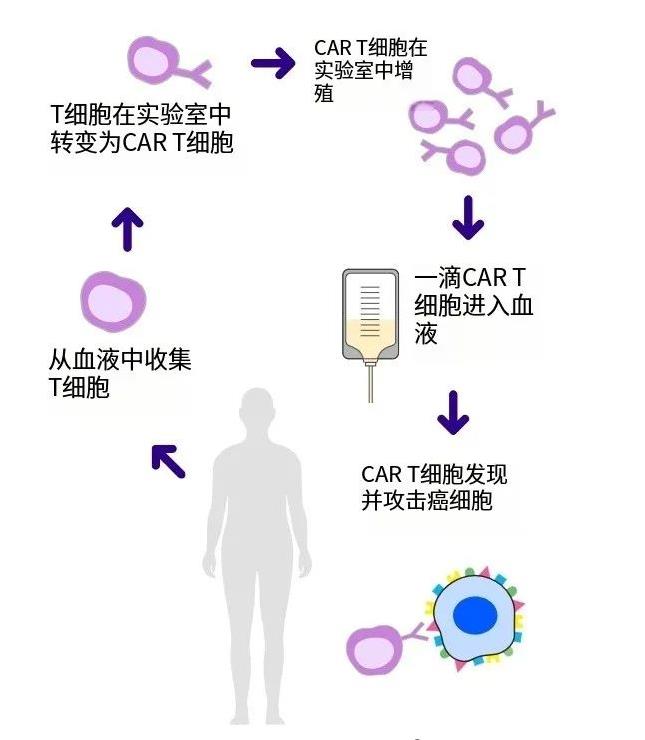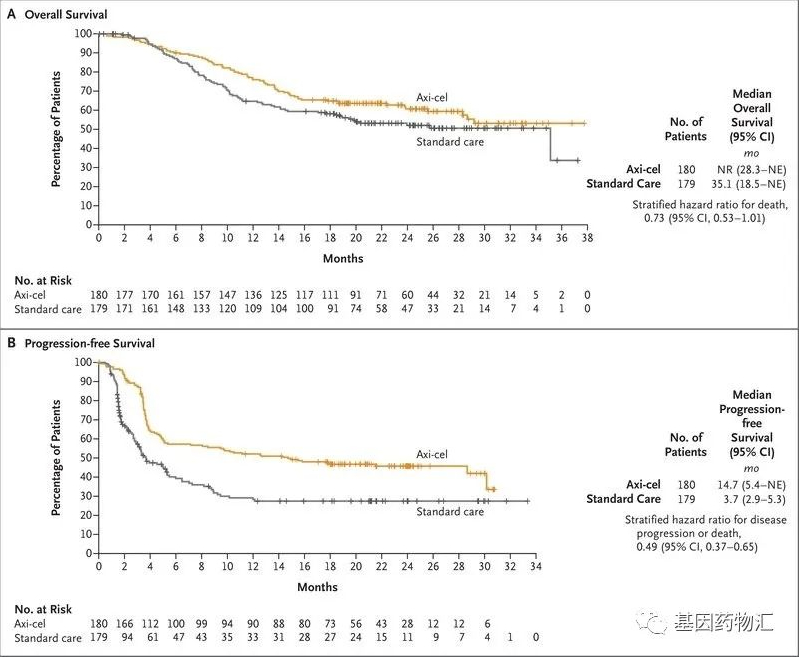
**BCMA CAR-T Therapy Paves New Path for Treating High-Risk Multiple Myeloma** Multiple Myeloma (MM) is a malignant tumor originating from plasma cells, known for its complex biology and treatment resistance, presenting significant challenges to patients’ survival. For high-risk MM, especially in cases of relapse and refractory disease after multiple lines of therapy, treatment options become Read More