The latest research on CAR-T Cell AML therapy
The latest research on CAR-T Cell AML therapy
Groundbreaking News! Dr. Lu Daopu’s Medical Team Publishes Latest Research in Blood Journal: Nanobody-based CD7 CAR-T Therapy for Acute Myeloid Leukemia
Recently, Dr. Lu Peihua, director of Lu Daopu Hospital, as the first author and corresponding author, published a research paper titled “Nanobody-based Naturally Selected CD7-Targeted Chimeric Antigen Receptor T Cell Therapy for Acute Myeloid Leukemia” in the authoritative international hematology journal Blood (IF=21). This research achievement marks another major breakthrough in the treatment of acute myeloid leukemia (AML).
Blood
In this study, Dr. Lu Peihua and her team innovatively adopted a nanobody-based CD7 CAR-T therapy for acute myeloid leukemia. The research results achieved significant progress not only in tumor killing and safety but also paved a new path for the treatment of refractory and relapsed acute myeloid leukemia.
Research Background
Refractory or relapsed (R/R) acute myeloid leukemia (AML) has a relatively poor prognosis, even in patients who have received allogeneic hematopoietic stem cell transplantation (allo-HSCT), necessitating new therapies. Approximately 30% of AML patients express CD7 on their malignant cells. Our center has previously published related articles demonstrating the significant efficacy and good safety of naturally selected CD7 CAR-T (NS7CAR-T) therapy in T-cell lymphoblastic leukemia/lymphoma.
The traditional targeting domain of chimeric antigen receptors (CARs) relies on the single-chain variable fragment (scFv) from the variable region of monoclonal antibodies (mAbs). Recently, an alternative approach using the single variable domain of camelid heavy-chain antibodies, known as nanobodies, has become increasingly important. Nanobodies have a molecular size (15kDa) approximately one-tenth that of human IgG molecules. While traditional mAbs require six complementarity-determining regions (CDRs) to bind antigens, nanobodies can maintain considerable affinity and specificity with only three CDRs. Furthermore, due to their similarity to the human VH gene family III, nanobodies exhibit lower immunogenicity than murine mAbs. Notably, a mature surface display platform also facilitated the generation of multiple nanobodies targeting different epitopes of the same antigen, a capability typically more limited for traditional mAbs. Moreover, particularly relevant is that nanobody-based CAR-T cells have exhibited enhanced effector cytokine release. We adopted the nanobody technology to produce NS7CAR-T cells. In this article, we report the preclinical data of the Nanobody-based NS7CAR-T product and the safety and efficacy results of Nanobody-based NS7CAR-T therapy for difficult-to-treat/relapsed CD7-positive acute myeloid leukemia (AML) in a phase I clinical trial (https://clinicaltrials.gov NCT04938115).
Research Methods
The nanobody-based dVHH NS7CAR contains the coding regions of two anti-CD7 nanobodies (VHHs) connected by a (G4S)5 linker, as well as the CD8α hinge region, CD8α transmembrane domain, and a second-generation CAR scaffold with intracellular co-stimulatory domains 4-1BB and CD3ζ. Peripheral blood (PB) mononuclear cells were obtained through leukapheresis, T cells were isolated and transduced with lentivirus. The second-generation CD7CAR consists of an anti-CD7 single-chain antibody, an IgG4 hinge region, a CD28TM transmembrane structure domain, intracellular co-stimulatory domains 4-1BB and CD3ζ, and a truncated EGFR protein linked by T2A. All patients received fludarabine (30 mg/m²/d) and cyclophosphamide (300 mg/m²/d) intravenously 3 days before CAR-T cell infusion. The median time from collection to CAR-T cell infusion was 15 days.
Research Results
Data from the mouse trial of car-t in the treatment of AML
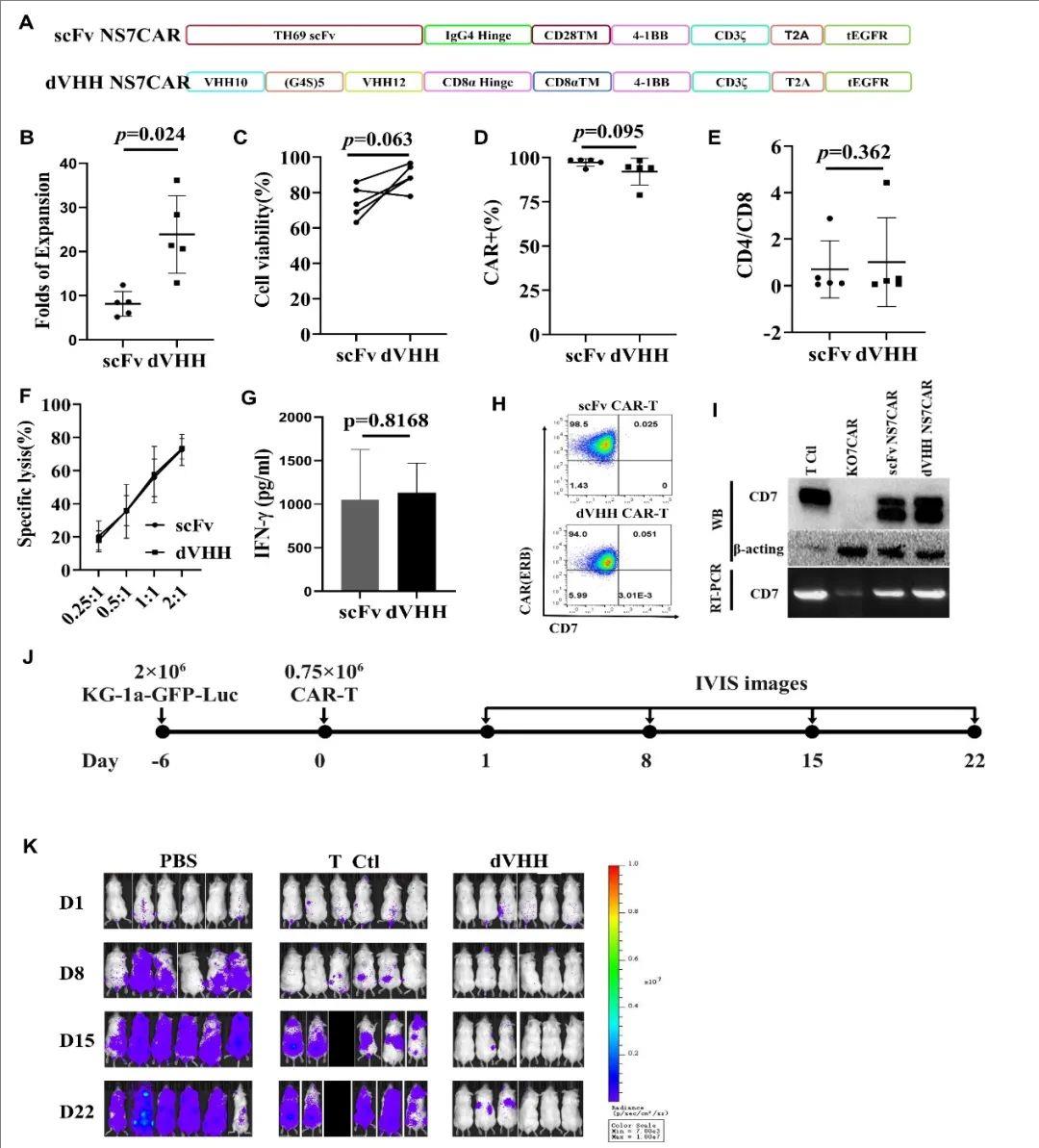
First, the preclinical study compared the structures of dVHH NS7CAR-T and traditional scFv NS7CAR, as shown in the figure (Figure A). The study found that dVHH NS7CAR-T cells showed stronger expansion ability than scFv NS7CAR-T cells after approximately two weeks in culture (23.9 folds vs. 8.1 folds, P=0.024) (Figure B).
At different effector-to-target cell ratios, scFv and dVHH NS7CAR-T cells exhibited similar anti-malignant cell function against KG1a target cells (Figure F). Flow cytometry (FCM), reverse transcription-polymerase chain reaction (RT-PCR), and Western blotting (WB) analyses showed that both scFv NS7CAR-T and dVHH NS7CAR-T cells did not exhibit FCM-detectable CD7 expression on their cell surface (Figure H), but significant CD7 mRNA and protein levels were detected by RT-PCR and WB (Figure I). dVHH NS7CAR-T cells displayed potent anti-tumor activity (Figure K), exhibiting significant anti-tumor activity on day 8 post-infusion. In contrast, mice treated with PBS or control T cells showed rapid tumor growth (Figure J).
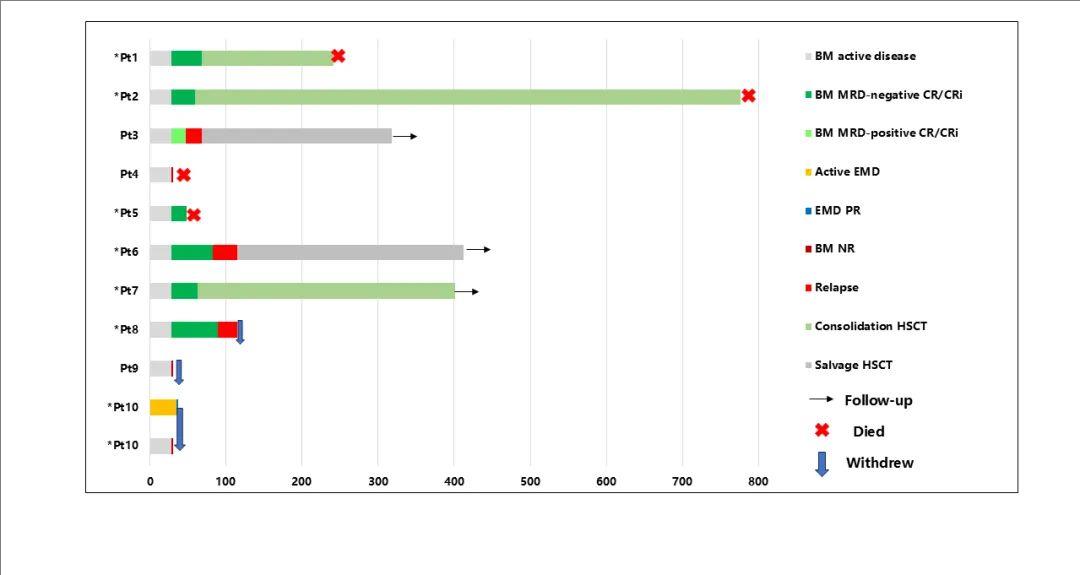
In this phase I clinical trial, 12 patients with CD7-positive R/R AML (CD7 expression >50%, bright intensity) were enrolled, and 10 patients ultimately received dVHH NS7CAR-T cell infusion, with 4 patients receiving 5×10⁵/kg and 6 patients receiving 1×10⁶/kg. The median age was 34 years (range 7-63 years), and the median bone marrow tumor burden at enrollment was 17.0% (range 2.0-72.7%). One patient had extramedullary disease (EMD). Prior to enrollment, patients had received a median of 8 (range 3-17) lines of treatment. Additionally, 7 patients had a history of hematopoietic stem cell transplantation, with a median interval from the last transplant to relapse of 12.5 months (range 3.5-19.5 months).
At 28 days after dVHH NS7CAR-T cell infusion, 7/10 (70%) patients achieved complete remission (CR) in the bone marrow, with 6 of them achieving MRD-negative CR. Three patients were non-responders (NR), one of whom with EMD achieved partial remission (PR) based on PET-CT evaluation on day 35. All NR patients exhibited CD7 antigen loss. The median follow-up time was 178 days (range 28-776 days). Among the 7 CR patients, 3 received consolidative second allogeneic hematopoietic stem cell transplantation approximately 2 months after CD7 CAR-T cell infusion. One patient remained in remission for 401 days of follow-up, while the other 2 patients died from non-relapse causes, renal failure, and infection at 241 days and 776 days, respectively. For the remaining 4 CR patients who did not receive bridging transplantation, 3 relapsed on days 47, 83, and 89 (all 3 patients lost CD7 expression at relapse), and 1 died from pulmonary infection.
Results of car-t treatment of AML in mice
Regarding safety, most patients (80%) experienced mild cytokine release syndrome (CRS), with 7 patients having grade I, 1 patient grade II, and 2 patients (20%) grade III CRS. No neurotoxicity occurred. Among the 7 patients with a history of transplantation, 1 patient (who relapsed approximately 100 days after the previous transplant) developed mild skin graft-versus-host disease after CAR-T therapy.
Conclusion
Our study demonstrates that dVHH NS7CAR-T therapy can induce initial complete remission in difficult-to-treat relapsed AML patients, even for those who had received multiple prior lines of treatment and relapsed after transplantation, with manageable safety of NS7CAR-T treatment. Consolidative allogeneic hematopoietic stem cell transplantation (allo-HSCT) still played a role in maintaining long-term remission in our trial following dVHH NS7CAR-T therapy.
CD7 antigen loss was a major issue in non-responding or relapsing patients, and we found that 5/6 patients with double CEBPA mutations in AML subsequently lost CD7 antigen, suggesting a poorer prognosis. This indicates that future clinical trials of CD7 CAR-T therapy for AML may need to consider excluding such patients.
Finally, to better evaluate the efficacy and safety of nanobody-based NS7CAR-T therapy for CD7-positive AML, further studies with larger patient numbers and longer follow-up are needed to confirm the findings.

Blood
Acute myeloid leukemia (AML) is a complex and highly heterogeneous hematological malignancy, and the limitations of current treatment approaches have posed significant challenges to achieving long-term remission and overall survival rates. Dr. Lu Peihua’s team has innovatively applied a naturally selected CD7-targeted nanobody-based chimeric antigen receptor T cell therapy in the treatment of AML, demonstrating excellent therapeutic effects and lower off-target toxicity. Their comprehensive data have been highly evaluated by international peers.
The publication of this achievement not only brings new hope to AML patients but also highlights the prominent academic position of the Lu Daopu Medical Team in the global field of hematology research. In the future, we will continue to delve into basic and clinical research in hematological diseases, continuously promoting the translation and application of scientific achievements to bring more good news to patients with blood disorders worldwide.
