Haematologica: The Best Response Rate of CD19 CAR-T Cell Treatment for Lymphoma was 77%
Haematologica: The Best Response Rate of CD19 CAR-T Cell Treatment for Lymphoma was 77%
CD19 CAR-T Cell Therapy in Different Subtypes of Relapsed/Refractory Large B-Cell Lymphoma (r/r LBCL)
CD19 CAR-T cell therapy has significantly improved the prognosis of patients with relapsed/refractory large B-cell lymphoma (r/r LBCL), including diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), and LBCL transformed from follicular lymphoma (t-FL) or non-follicular lymphoma (t-NFL).
The efficacy of CD19 CAR-T therapy in r/r LBCL has been confirmed in long-term follow-up of registration trials and multiple large real-world retrospective cohorts. However, due to inclusion criteria and sample size limitations, the clinical benefits of CAR-T in different histological subtypes remain unclear. Subtype-specific CAR-T outcome data are crucial to understand the relative benefit of CAR-T compared to alternative therapies, such as CD20 XCD3 bispecific antibodies, in each subgroup, thereby guiding decision-making in routine practice.
Recently, the journal Haematologica published an article reporting the outcomes of 760 patients who received CD19 CAR-T cell therapy, stratified by histological subtype, from a large national database in the UK.

Study Results
As part of a National Service Evaluation, the study included 760 consecutive patients with r/r LBCL who received approval for axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) as ≥ third-line treatment at 12 CAR-T centers from December 2018 to October 2022.
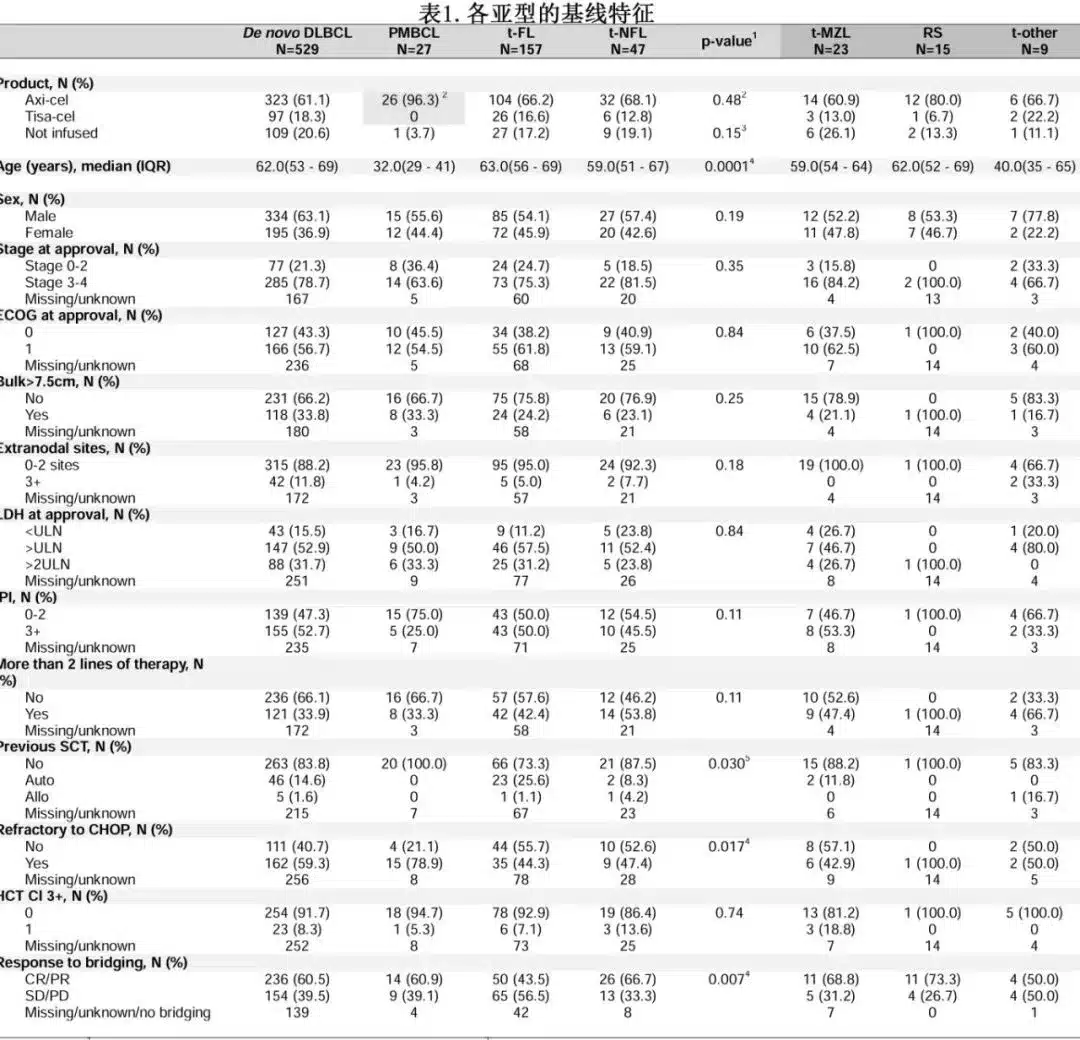
Among the 760 patients, 529 (70%) had primary DLBCL, 27 (4%) had PMBCL, 157 (21%) had t-FL, and 47 (6%) had t-NFL [including 23 t-marginal zone lymphoma (MZL), 15 Richter’s syndrome (RS), 5 t-nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), and 4 t-lymphoplasmacytic lymphoma (LPL)].
The baseline characteristics between the t-NHL group and the primary DLBCL group were not significantly different. PMBCL patients were significantly younger, and t-FL patients differed significantly from primary DLBCL patients in terms of CHOP-refractory disease and bridging remission (Table 1). Leukapheresis was performed in 720 patients (94.7%), and 614 patients (81%) received CAR-T cell infusion, with similar rates across subgroups. Among the 614 infused patients, 485 received axi-cel, and 129 received tisa-cel. Of the leukapheresed patients, 89.9% received bridging therapy.
The median follow-up time after CAR-T cell therapy approval was 18.2 months. The best overall response rate (ORR) was 77% (57% CR), with no significant differences between subgroups, but t-FL showed a trend toward better remission (ORR 84%/CR 70%; P=0.054).
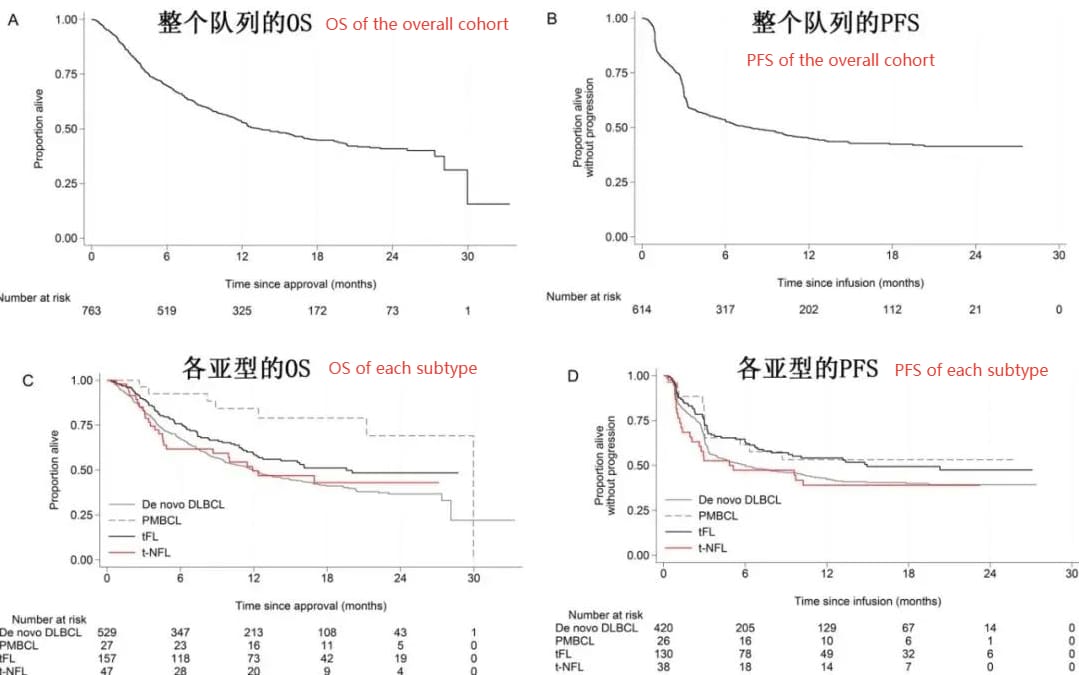
The 12-month progression-free survival (PFS) rates were 53% for PMBCL, 42% for primary DLBCL, 54% for t-FL, and 39% for t-NFL; the 12-month overall survival (OS) rates in the intention-to-treat (ITT) population were 84%, 50%, 58%, and 50%, respectively (Figure 1).
There were no significant differences in PFS or OS among the t-NFL subtypes (PFS: RS vs. t-MZL 0.80; t-others vs. t-MZL 0.51; RS vs. t-others 0.64; p=0.49, OS: RS vs. t-MZL 1.06; t-others vs. t-MZL 0.67; RS vs. t-others 0.63; p=0.79).
In both the ITT and infused populations, t-FL had significantly better PFS than primary DLBCL (HR=0.75; p=0.043); PMBCL and t-FL had significantly better OS (for the infused population: PMBCL HR=0.34, p=0.005; t-FL HR=0.73, p=0.017).
5% of patients experienced grade ≥3 cytokine release syndrome (CRS), and 15% experienced grade ≥3 immune effector cell-associated neurotoxicity syndrome (ICANS), with similar rates across subgroups. No significant differences were observed in the use of tocilizumab and steroids, ICU admission, or non-relapse mortality rates.
Summary
This study provides evidence for the clinical benefit of CAR-T in rare subtypes of r/r LBCL (such as t-NFL) and further demonstrates that outcomes are particularly favorable in PMBCL and t-FL patients, highlighting the important role of CD19 CAR-T cell therapy relative to other treatment options. However, these findings should be confirmed in larger datasets.
Content Source:上海细胞治疗工程技术研究中心
