BLOOD Publishes Long-Term Follow-Up Data from the ELARA Study: Tisagenlecleucel (Kymriah) Provides Durable Responses in Patients with Relapsed/Refractory Follicular Lymphoma (R/R FL)
BLOOD Publishes Long-Term Follow-Up Data from the ELARA Study: Tisagenlecleucel (Kymriah) Provides Durable Responses in Patients with Relapsed/Refractory Follicular Lymphoma (R/R FL)
Currently, there is no clearly defined standard of care (SOC) for patients with relapsed/refractory follicular lymphoma (R/R FL). Patients with high-risk factors, such as disease progression within 24 months after first-line immunochemotherapy (POD24) or high tumor burden at baseline, have a poor prognosis and an increased risk of mortality. Clinically, there is an urgent need for effective and safe drugs to further meet the treatment needs of FL.
Tisagenlecleucel (Kymriah, Tisa-Cel) is a CD19 CAR-T cell therapy approved in the United States, European Union, and Japan for the third-line treatment of adult R/R FL. Preliminary analysis of the ELARA study confirmed its significant therapeutic potential in R/R FL. Recently, the long-term follow-up data [1] from this study were published in the prestigious hematology journal BLOOD. This article summarizes the findings as follows.

Figure 1. Research snapshot
Long-term follow-up results announced, Tisagenlecleucel balances efficacy and safety
The ELARA study is a single-arm, global, multicenter, open-label phase II study that explored the efficacy and safety of Tisagenlecleucel in R/R FL. As of March 29, 2022, a total of 98 patients with R/R FL were enrolled, and 97 patients (1-3A grade) received Tisa-Cel infusion treatment at a dose of 0.6×108-6×108 CAR-T cells, with bridging chemotherapy allowed.
In the previously reported preliminary analysis of the phase II ELARA trial (median follow-up of 17 months), Tisagenlecleucel demonstrated a high objective response rate (ORR=86%), complete response rate (CRR=69%), and durable responses [12-month progression-free survival (PFS) rate of 67%] in high-risk adult patients with R/R FL (including POD24 and high tumor burden). It also showed a favorable safety profile, with ≥ grade 3 cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) occurring in only 1% of patients. The recently announced long-term follow-up data further confirmed the feasibility of this drug.
1. ORR reached 86.2% after over 2 years of follow-up!
The median follow-up time in the study was 29 months. Efficacy analysis included 94 patients, and the median PFS, duration of response (DoR), overall survival (OS), and median time to next treatment (TTNT) were not reached. The estimated 24-month PFS rate for all patients was 57.4%, the estimated 24-month DoR rate was 66.4%, and the estimated 24-month TTNT rate was 69.7% (Figure 2).
The CRR and ORR were 68.1% (64/94) and 86.2% (81/94), respectively. For patients who achieved CR, the estimated 24-month DoR rate was 77.8%, and the estimated 24-month OS rate was 87.7%. Among the 31 patients who initially achieved partial response (PR), 14 converted to CR within 6 months after receiving Tisagenlecleucel infusion. Patients who achieved CR demonstrated better efficacy across all efficacy measures compared to the overall population. Twenty-five (31%) patients relapsed after achieving a response, with a median time to relapse of 121.5 days (range: 43 days to 635 days).
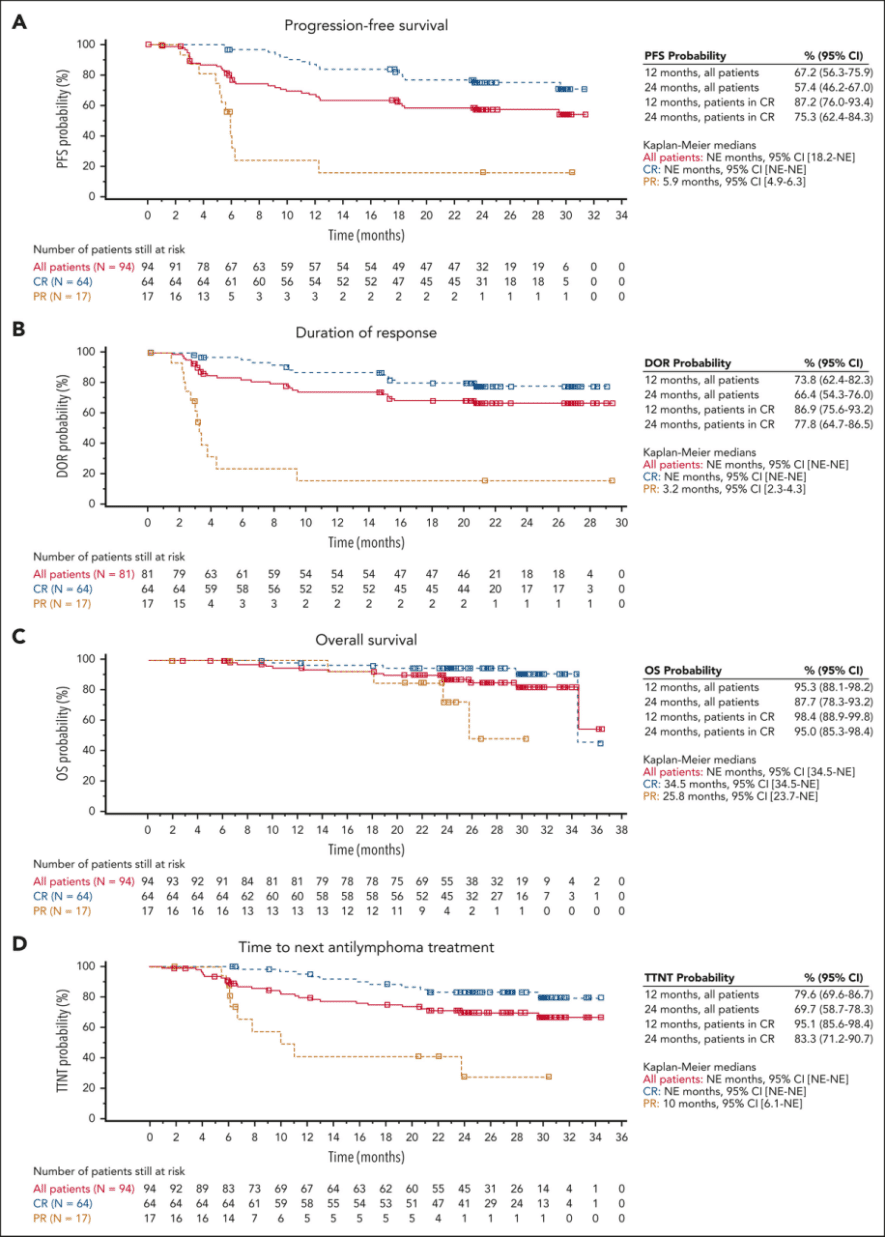
Figure 2. Kaplan-Meier curves for R/R FL patients
2. Promising efficacy in high-risk subgroups, safety profile consistent with previous reports
Tisagenlecleucel induced durable responses in all patients, including those with high-risk baseline disease characteristics, such as POD24 (ORR 82%; CRR 59%). Subgroup analysis showed consistent treatment effects across all subgroups, with no significant changes in CRR and ORR. The estimated 24-month PFS rates and DoR rates for patients with high and low tumor burden who achieved CR were 42.9%, 78.8%, and 42.9%, 81.8%, respectively.
Furthermore, in this long-term follow-up analysis, the safety profile of Tisa-Cel was consistent with the previously published preliminary analysis, with no new safety signals or treatment-related deaths observed, confirming the manageable safety profile of this drug.
Emerging potential to support personalized treatment
Currently, while treatment options for R/R FL patients are increasing, selecting personalized treatment to achieve the best prognosis remains a challenge, particularly for patients who relapse after CAR-T cell therapy. The extended follow-up of over 2 years from the ELARA study demonstrates that Tisagenlecleucel can provide significant clinical benefits and a manageable safety profile in adult patients with R/R FL, including those with high-risk features and currently limited effective treatment options, potentially offering a treatment choice.
Content Source:医学界血液频道
