CAR-T Lymphoma Therapy: A Paradigm Shift in Precision Cancer Immunotherapy
CAR-T Lymphoma Therapy: A Paradigm Shift in Precision Cancer Immunotherapy
Lymphoma is a malignant hematopoietic system disease that threatens human health. As one of the common malignant tumors, about 100,000 new cases of lymphoma are diagnosed in China each year, and the incidence rate shows an increasing trend year by year. Approximately 50-85% of common adult malignant lymphomas can be cured by standard first-line treatment. However, the treatment of refractory or relapsed lymphoma after initial remission has always been a formidable challenge, and the prognosis is generally poor.
The emergence of CD19-targeted CAR-T therapy has rekindled hope for patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. CAR-T cell therapy (Chimeric Antigen Receptor T cell therapy) refers to a method of collecting and genetically modifying the patient’s own T cells to produce CAR-T cells, which are then reinfused into the patient’s body to kill cancer cells. This is a globally advanced new precision immunocell targeting therapy.
Currently, CAR-T cell therapy is applicable to the treatment of B-NHL and relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL) in children and adults. Of course, more and more new target CAR-T cell therapies are being used for various tumors. Here, we will briefly discuss the application of CAR-T cell therapy in lymphoma.
I. The origin and process of CAR-T cell therapy
CAR-T cell therapy is undoubtedly a revolutionary change in the history of cancer treatment, and is considered a milestone event. CAR-T cell therapy began in 2012, when Emily Whitehead, a girl with relapsed and refractory acute lymphoblastic leukemia, became the first patient to receive CAR-T cell therapy. To date, she has achieved over 10 years of cancer-free survival, and CAR-T cells can still be detected in her body. She posts a photo on social media every year to commemorate the event, and has done so for 10 consecutive years.
Simply put, CAR-T therapy involves collecting T lymphocytes from the patient’s body, equipping the T cells with a CAR (chimeric antigen receptor) through genetic engineering, and culturing them into cytotoxic T cells specifically targeting tumor-specific antigens – CAR-T cells. After being reinfused into the patient’s body, the CAR-T cells proliferate and can precisely identify and kill tumor cells. For lymphoma, our target infusion dose is 106/kg, and clinically it generally ranges from 105/kg to 109/kg. Compared to the infusion dose, in vivo expansion is more important for efficacy. After infusion, CAR-T cells can expand 1000 to 10000 times in the body, with a median peak expansion occurring between 10 and 14 days, and a median in vivo persistence of 3 months (some lasting >1 year). Studies have shown that expansion may be related to efficacy, as patients who respond to CART treatment generally exhibit high CART expansion around day 10.
II. Efficacy of CAR-T cell therapy
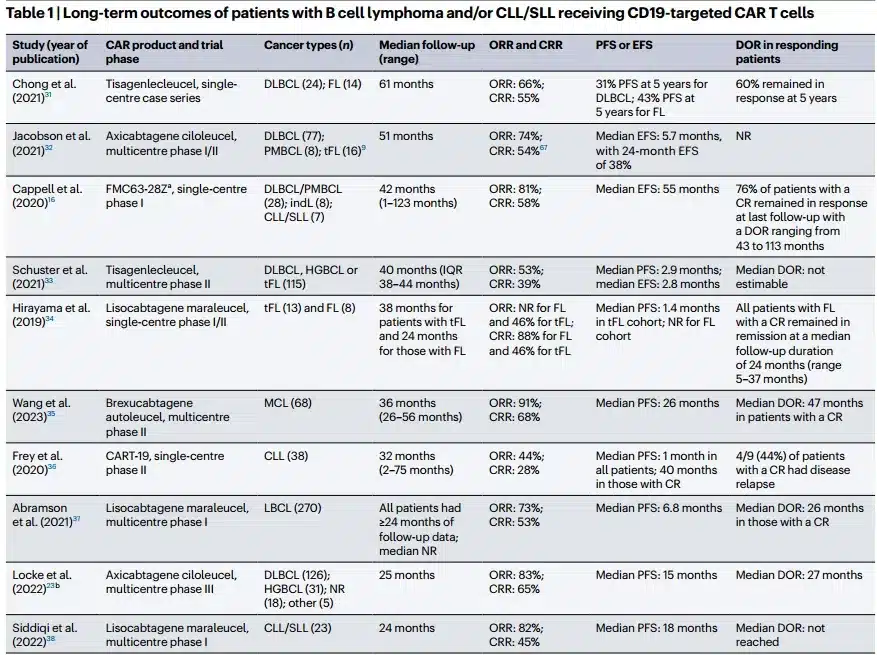
The image is from the literature [4].
There are 10 clinical trials of CD19-targeted CAR-T therapy in patients with relapsed/refractory B-cell lymphoma and chronic lymphocytic leukemia, providing ≥1 year of follow-up data (range 24-123 months). The analysis shows that the overall response rate of CD19 CAR-T therapy is 44-91%, and the complete remission rate (CR) is 28-68%. All clinical trials reported a subgroup of patients who maintained deep remission for ≥2 years after infusion without any consolidation therapy.
Currently, CAR-T therapy is used to treat refractory and relapsed patients, and should be considered as early as possible after failure of two lines of chemotherapy. Patients with normal organ function, ability to self-care, stable disease status, and low tumor burden are suitable for CAR-T therapy, and treatment should not be delayed until the disease progresses to a severe condition. Clinically, doctors will also assess the patient’s sensitivity to chemotherapy at an early stage. Patients with poor prognostic factors at onset, those who do not respond to standard first-line chemotherapy, those who respond to second-line chemotherapy but progress within one cycle, and those who relapse shortly after autologous transplantation are considered chemotherapy-insensitive, and immunotherapy should be considered as early as possible.
According to Dr. Frederick Locke, Co-Director of the Blood and Marrow Transplant and Cellular Immunotherapy Program, there are three situations in which patients should be referred for CAR-T cell therapy as early as possible:
1. The first situation is when the first-line treatment regimen is ineffective, and the patient still has positive disease on a PET scan after three cycles;
2. The second situation is when the patient has completed first-line treatment but still has residual tumor;
3. The third situation is when the patient relapses within 12 months after completing first-line treatment.
III. Overview of CAR-T cell therapies approved for lymphoma treatment
United States:
Kymriah (Tisagenlecleucel): B-cell precursor acute lymphoblastic leukemia; relapsed or refractory diffuse large B-cell lymphoma.
Yescarta (Axicabtagene ciloleucel): Relapsed or refractory diffuse large B-cell lymphoma; relapsed or refractory follicular lymphoma.
Tecartus (Brexucabtagene autoleucel): Relapsed or refractory mantle cell lymphoma.
Breyanzi (Lisocabtagene maraleucel): Relapsed or refractory diffuse large B-cell lymphoma.
China:
Axi-Cel (Axicabtagene Ciloleucel): Relapsed or refractory diffuse large B-cell lymphoma.
Relma-cel (Relmacabtagene Autoleucel): Relapsed or refractory diffuse large B-cell lymphoma.
IV. Adverse reactions of CAR-T cells
While CAR-T therapy undoubtedly brings new hope for cancer treatment, it also has severe adverse reactions, primarily cytokine release syndrome (CRS) and neurotoxicity.
CRS occurs because the large number of infused CAR-T cells activates the immune system, leading to the release of excessive inflammatory cytokines and causing severe adverse reactions. Clinical manifestations of CRS include fever, chills, nausea, fatigue, muscle pain, capillary leakage, generalized edema, flushing, hypotension, oliguria, tachycardia, heart failure, dyspnea, respiratory failure, liver failure, and kidney damage.
The cause of neurotoxicity is currently unclear but may be related to CRS. Clinical manifestations include headache, delirium, partial loss of language ability, delayed response, seizures, coma, and even death due to brain edema.
Allergic reactions are also foreseeable adverse reactions, primarily manifesting as rash, while other reactions cannot be distinguished from CRS. The high cost of CAR-T therapy is also a major challenge, making it unaffordable for most cancer patients.
Currently, cellular immunotherapy is gaining momentum. From first-generation CAR-T, second-generation CAR-T, third-generation CAR-T, to fourth-generation CAR-T; immune cell types include CAR-T, CAR-NK, TCR-T, UCAR-T, FAST-CAR, and more; from single targets to multiple targets; and from hematological malignancies to solid tumors. A recent study published in Blood showcased a bispecific CD19/CD22 CAR-T product, AUTO3, in patients with relapsed/refractory LBCL, injecting new vitality into the field. With the continuous development of molecular biology techniques, more breakthroughs will be made in the design of CAR molecules, and safer and more effective universal CAR-T therapies will be developed to benefit more cancer patients in the future.
References
[1]SCHUSTER S J,SVOBODA J,CHONG E A, et al. Chimeric antigen receptor T Cells in refractory B-Cell lymphomas [J].New England Journal of Medicine, 2017, 377(26): 2545-54.
[2]Chimeric Antigen Receptor-TCells: A Pharmaceutical Scope. Front Pharmacol. 2021.08.20; 12: 720692.
[3].CAR-T design: Elements and their synergistic function. EBioMedicine. 2020;58:102931.
[4]long termTCR Redirected T Cells for Cancer Treatment: Achievements, Hurdles,and Goals. Front Immunology. 2020 Sep 3;11:1689.
[5]RODDIE C,LEKAKIS L J,MARZOLINI M A V, et al.Dual targeting of CD19 and CD22 with bicistronic CAR-T cells in patients with relapsed/refractory large B-cell lymphoma [J]. Blood, 2023, 141(20): 2470-82.
Content Source:安阳市肿瘤医院订阅号
