CAR-T Mantle Cell Lymphoma, Tecartus (Brexucabtagene Autoleucel) is Safe and Effective as the Standard Treatment for Mantle Cell Lymphoma
CAR-T Mantle Cell Lymphoma, Tecartus (Brexucabtagene Autoleucel) is Safe and Effective as the Standard Treatment for Mantle Cell Lymphoma
On February 8, 2023, JCO Online published the clinical results from the US Lymphoma CAR-T Alliance on Brexucabtagene Autoleucel (Tecartus, brexu-cel) as a standard treatment for relapsed/refractory mantle cell lymphoma. Let’s take a look with the editor.

Research Methods
Mantle cell lymphoma (MCL) is a mature B-cell lymphoma with clinical manifestations ranging from indolent to aggressive. The treatment of relapsed/refractory MCL has always been a clinical challenge, and the emergence of chimeric antigen receptor T-cell (CAR-T) immunotherapy has brought a ray of hope.
Brexucabtagene Autoleucel is the first FDA-approved autologous CAR-T cell therapy for relapsed/refractory MCL. This approval was based on the results of the single-arm phase II ZUMA-2 clinical trial, but ZUMA-2 had strict inclusion criteria regarding complications and prior treatment history. In clinical practice, all adult patients with relapsed/refractory MCL can receive Brexucabtagene Autoleucel treatment. Therefore, the safety and efficacy of Brexucabtagene Autoleucel in actual standard treatment need further investigation.
Research Methods
This was a prospective study that enrolled patients who underwent leukapheresis for commercial Brexucabtagene Autoleucel treatment at 16 institutions in the United States between August 1, 2020, and December 31, 2021. Patient data were collected according to standard guidelines for analysis of response, treatment outcomes, and toxicity after drug administration.
Results
As of December 31, 2021, 189 patients completed leukapheresis, with a median age of 67 years, and 76% were male. Among these patients, 149 (79%) did not meet the ZUMA-2 inclusion criteria.
Eventually, 128 patients (68%) received bridging therapy, and the overall response rate (ORR) of the bridging therapy was evaluated in 95 patients, with an ORR of 33% (6% complete response [CR], 27% partial response [PR]). 21 (11%) patients did not receive Brexucabtagene Autoleucel infusion due to death (n=9), manufacturing failure (n=7), disease progression (n=2), organ dysfunction (n=1), CR after bridging therapy (n=1), and patient refusal (n=1). The remaining 168 patients completed Brexucabtagene Autoleucel infusion (Figure 1).
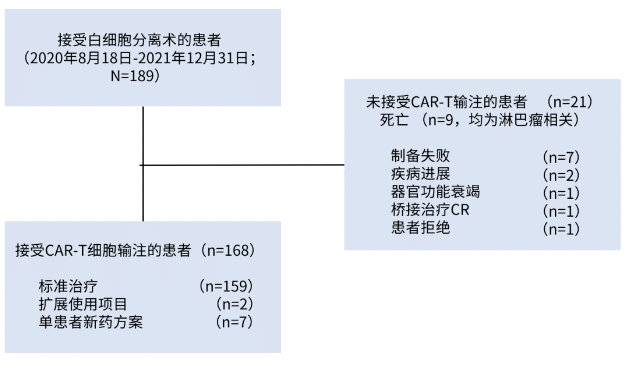
Figure 1. Study flow diagram
Among the patients who received Brexucabtagene Autoleucel infusion, the incidence of cytokine release syndrome (CRS) was 90% (8% ≥ grade 3), and the incidence of immune effector cell-associated neurotoxicity syndrome (ICANS) was 61% (32% ≥ grade 3), similar to the results of the ZUMA-2 study.
The median time to CRS onset was 4 days after treatment (range: 0-13 days), with a median duration of 5 days (range: 1-33 days). The median time to ICANS onset was 6 days after treatment (range: 1-18 days), with a median duration of 6 days (range: 1-144+ days). Drugs used to treat CRS and/or ICANS included tocilizumab (77%), corticosteroids (69%), anakinra (17%), and siltuximab (3%).
With a median follow-up of 14.3 (95% CI, 12.7 – 15.9) months after infusion, the best ORR among all patients who received Brexucabtagene Autoleucel treatment was 90%, with a CR rate of 82% and a PR rate of 8% (Figure 2). Among patients who achieved disease response, the median time to best response was 30 days (range: 16-193 days). The median duration of response (DOR) was 17.2 months (95% CI, 14.4-not estimable [NE]), and the 6-month and 12-month sustained response rates were 75% (95% CI, 68-82) and 65% (95% CI, 56 – 72), respectively. The median progression-free survival (PFS) after brexu-cel infusion was 16.4 months (95% CI, 12.7-NE), with 6-month and 12-month PFS rates of 69% (95% CI, 61-75) and 59% (95% CI, 51-66), respectively.
During the follow-up period, the median overall survival (OS) after Brexucabtagene Autoleucel infusion was not reached, with 6-month and 12-month OS rates of 86% (95% CI, 79 – 90) and 75% (95% CI, 67 – 81), respectively. The non-relapse mortality rates at day 30, day 90, and 1 year were 2.4% (95% CI, 0.8 – 5.6), 4.8% (95% CI, 2.2 – 8.8), and 9.1% (95% CI, 5.3 – 14.1), respectively. Among all patients who underwent leukapheresis, the median PFS after leukapheresis was 17.3 months (95% CI, 10.7-NE), and the median OS was not reached.
In univariate analysis, high-risk simplified Mantle Cell Lymphoma International Prognostic Index (MIPI), high Ki-67 expression, TP53 alteration, complex karyotype, and blastoid/pleomorphic variant were associated with shorter PFS after Brexucabtagene Autoleucel infusion. High-risk simplified MIPI, TP53 alteration, and complex karyotype were also associated with shorter OS.
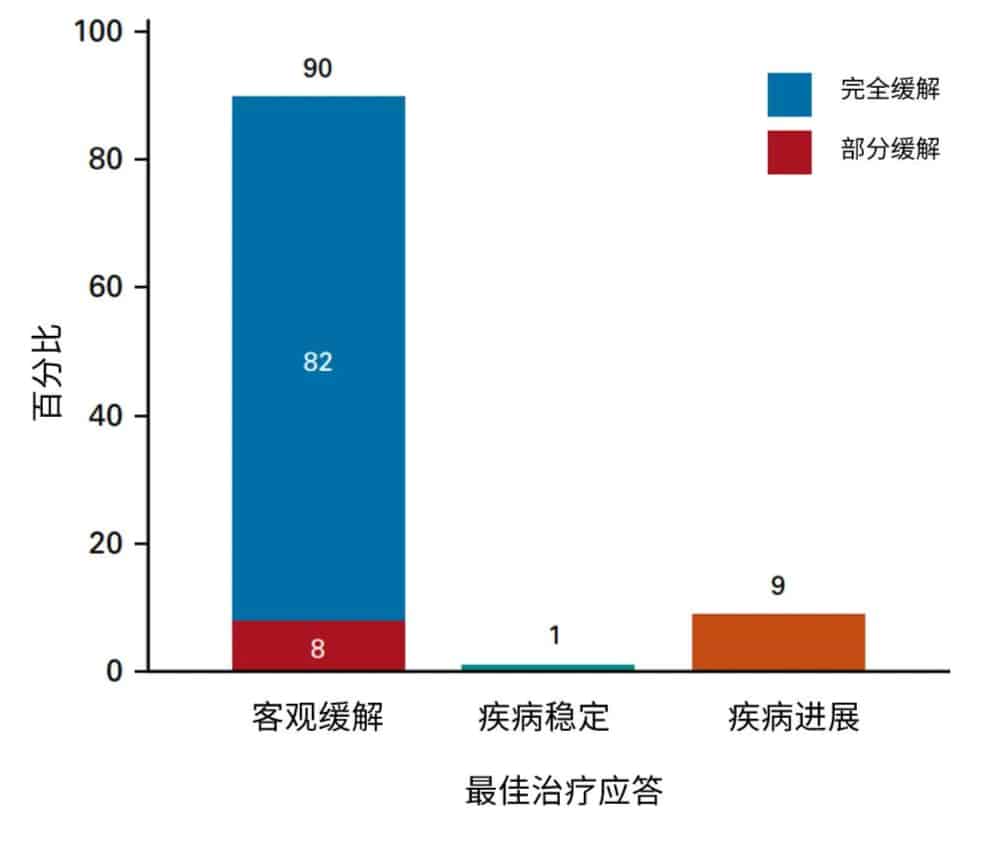
Figure 2. Best response after Brexucabtagene Autoleucel treatment
In subsequent analyses, the researchers found that prior treatment with Bruton’s tyrosine kinase (BTK) inhibitors did not affect patients’ PFS or OS. Whether or not patients met the ZUMA-2 inclusion criteria did not affect PFS, but patients who did not meet the ZUMA-2 criteria due to disease status or comorbidities had worse OS. Compared to patients who did not receive bridging therapy, those who received bridging therapy had higher levels of Ki-67 expression and blastoid/pleomorphic variant, but there were no statistically significant differences in PFS or OS between the two groups, and the response to bridging therapy was also not associated with PFS or OS.
Patients exposed to bendamustine within 6 months before leukapheresis not only had a higher rate of failure to receive Brexucabtagene Autoleucel infusion due to manufacturing failure or other reasons but also had lower ORR and CR rates.
Discussion
This study, along with other real-world data, further confirms the safety and efficacy of Brexucabtagene Autoleucel in routine treatment of patients with relapsed/refractory MCL. Compared to the ZUMA-2 study, the current study had a higher proportion of patients with high-risk simplified MIPI (69% vs. 59%), blastoid/pleomorphic variant (43% vs. 31%), and TP53 alteration (48% vs. 17%), as well as more patients with comorbidities. The success rate of Brexucabtagene Autoleucel manufacturing and safety data were similar to ZUMA-2, but the incidence of grade 3 CRS was lower in this study, possibly due to earlier use of tocilizumab and corticosteroids.
Furthermore, the ORR in this study was comparable to ZUMA-2 (90% vs. 91%), but the CR rate was slightly higher, possibly because more patients received bridging therapy in this study. The 12-month DOR and PFS after treatment in this study were also comparable to ZUMA-2, which is encouraging considering the higher proportion of high-risk features and comorbidities in this study.
Previous studies have shown that bendamustine may impair T-cell function, thus affecting the preparation and function of CAR-T cells. Similar results were observed in this study and ZUMA-2, where bendamustine exposure led to higher rates of CAR-T manufacturing failure and shorter PFS and OS for patients.
References
Wang Y, Jain P, Locke FL, et al. Brexucabtagene Autoleucel for Relapsed or Refractory Mantle Cell Lymphoma in Standard-of-Care Practice: Results From the US Lymphoma CAR T Consortium [published online ahead of print, 2023 Feb 8]. J Clin Oncol. 2023;JCO2201797.
Content Source:聚焦CA
