Study on the Therapeutic Effect of Mantle Cell Lymphoma CAR-T Combined with Ibrutinib
Study on the Therapeutic Effect of Mantle Cell Lymphoma CAR-T Combined with Ibrutinib
For patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL), the efficacy of CD19-targeted CAR-T cells is high; however, there is a risk of relapse and often significant toxicity. Here, we summarize the results of the phase II TARMAC trial (NCT04234061) published by Minson et al. in Blood, which investigated the efficacy of time-limited Ibrutinib in combination with CTL019 CAR-T cells for the treatment of R/R MCL patients.
Methods
This ongoing phase II study enrolled patients with R/R MCL who had received ≥1 prior line of treatment. Ibrutinib was initiated at least 7 days prior to leukapheresis and continued for at least 6 months after CAR-T cell infusion, driven by CAR-T cell persistence. The primary endpoint was the complete response rate (CR) at 4 months after CAR-T cell infusion. Secondary endpoints included safety, overall response rate, MRD negativity rates at months 1, 4, 6, 9, and 12, progression-free survival (PFS), and overall survival; responses were also analyzed by TP53 mutation status.
Results
The study enrolled 20 patients: 75% male; median age 66 years; median time from study enrollment to CAR-T cell infusion and CAR-T cell production was 60 days and 49 days, respectively; 50% of patients had prior Bruton’s tyrosine kinase inhibitor (BTKi) exposure, 45% were BTKi-refractory, and 15% had received >1 BTKi. Post-infusion, the 4-month endpoint CR rate was high, with a similar overall response rate, and 70% MRD negativity was observed (Figure 1).
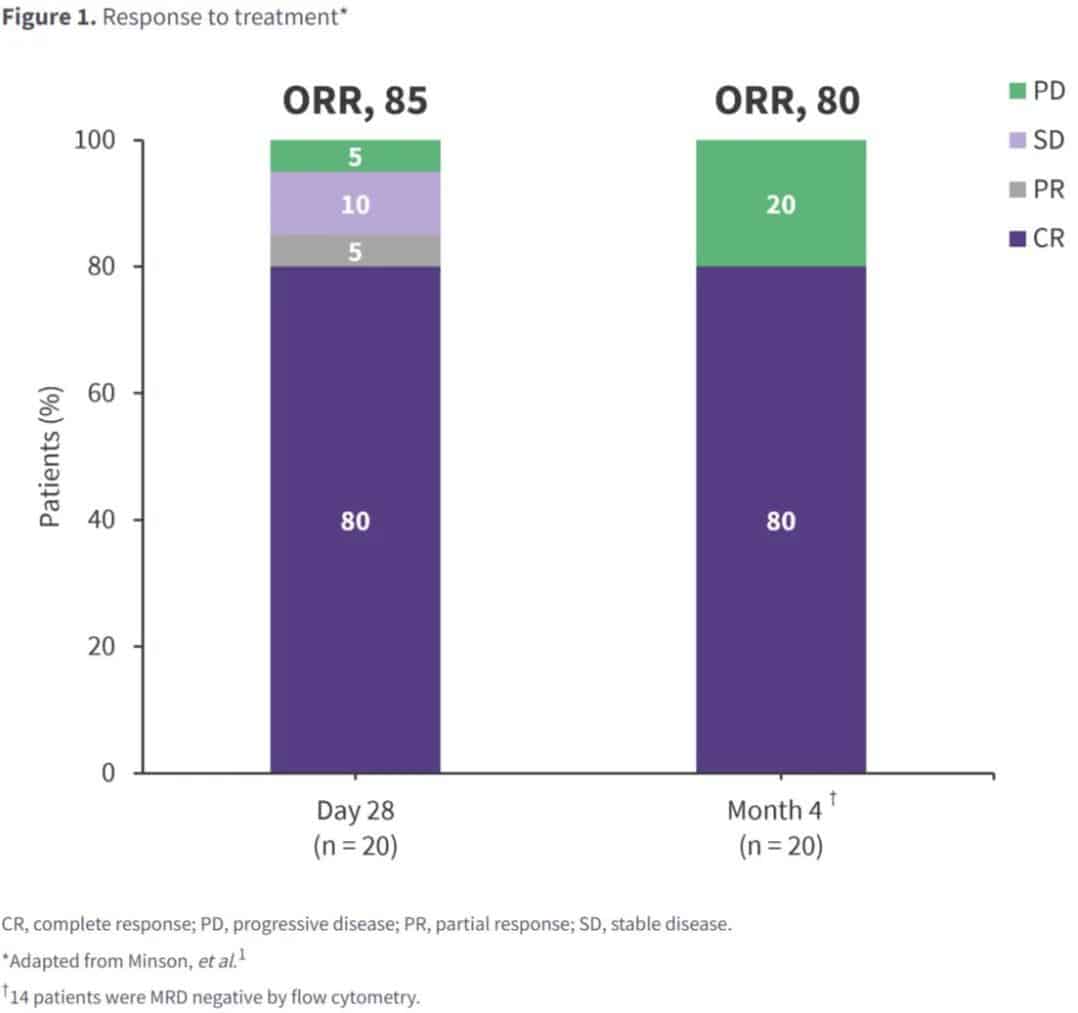
At a median follow-up of 13 months, the median PFS was not reached, with an estimated 12-month PFS and overall survival of 75% and 100%, respectively. Deep and durable responses were observed in high-risk subgroups, including patients with prior BTKi exposure or TP53 mutations. Depth of response was associated with robust CAR-T cell expansion and a less-exhausted baseline T-cell phenotype. All patients reported any-grade adverse events: 15 patients experienced CRS (grades 1-2, n=12; grade 3, n=3). Two patients experienced reversible grade 1-2 neurotoxicity.
Key Conclusions
CAR-T cells combined with time-limited Ibrutinib for R/R MCL patients demonstrated high CR and molecular MRD negativity rates, irrespective of prior BTKi exposure and TP53 mutation status. A less-exhausted baseline T-cell phenotype was associated with robust CAR-T cell expansion, leading to positive responses, but further study is needed to guide strategies to improve the safety and efficacy of BTKi and CAR-T cell combination in this patient population.
Content Source:医药学习天地
