Real world data of 1297 cases of Axicabtagene Ciloleucel DLBCL Treated in the United States
Real world data of 1297 cases of Axicabtagene Ciloleucel DLBCL Treated in the United States
Axi cel real world research
Axicabtagene ciloleucel (axi-cel) is an autologous CD19-directed chimeric antigen receptor (CAR) T-cell therapy that received FDA approval in 2017 for the treatment of adult patients with relapsed or refractory (r/r) large B-cell lymphoma (LBCL) after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), high-grade B-cell lymphoma, and transformed follicular lymphoma (TFL). The approval was based on the primary analysis of the ZUMA-1 pivotal trial; in the subsequent 2-year follow-up analysis, 83% of patients treated with axi-cel achieved an overall response, 58% achieved a complete response (CR), and the median overall survival (OS) was not reached.
As clinical trials typically have stringent eligibility criteria, the efficacy and safety outcomes observed in trials may not be replicated in real-world medical practice, where patients may have additional comorbidities or complications that would have led to exclusion from the trial. Studies on axi-cel have shown that even patients who did not meet the ZUMA-1 eligibility criteria had comparable efficacy and safety outcomes to the ZUMA-1 results, maintaining overall response rates and toxicity profiles.
To better understand the efficacy and safety outcomes of the commercialized axi-cel, Professor Marcelo C. Pasquini from the Medical College of Wisconsin and colleagues conducted a long-term follow-up study using the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The article was recently published in Transplantation and Cellular Therapy, the official journal of the American Society for Transplantation and Cellular Therapy (ASTCT).

Study Results
This analysis included 1,297 eligible patients from 78 clinical trial sites with a minimum follow-up of 6 months, comprising 739 patients who did not meet the ZUMA-1 eligibility criteria and 558 who did. The study did not exclude patients with pre-existing end-organ impairment and collected comorbidities categorized by the hematopoietic cell transplantation-comorbidity index (HCT-CI). All study data were derived from clinical, laboratory, and diagnostic assessments performed during routine medical practice.
Patient Characteristics
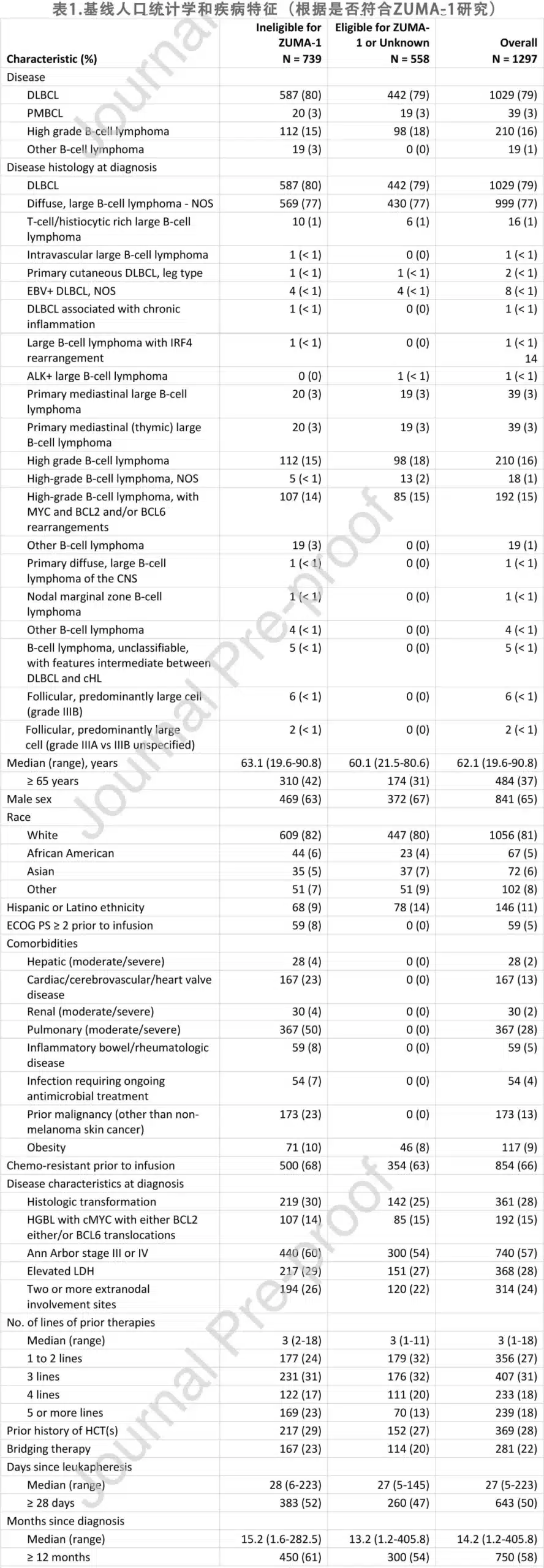
The baseline characteristics of the 1,297 patients are summarized in Table 1, including 1,029 (79%) with DLBCL, 39 (3%) with PMBCL, and 210 (16%) with high-grade B-cell lymphoma; among the high-grade B-cell lymphomas, 192 (15%) were high-grade B-cell lymphoma with MYC and BCL-2 and/or BCL-6 rearrangements. 361 (28%) patients had transformed large B-cell lymphoma. 739 (57%) patients were deemed ineligible for the ZUMA-1 trial, including 1 patient with primary central nervous system (CNS) lymphoma and 18 patients with secondary CNS involvement. 22 patients had histologic transformation from chronic lymphocytic leukemia (CLL). At the time of infusion, 5% of patients had an ECOG performance status ≥2. Among the comorbidities, 28% of patients had moderate to severe lung disease, 13% had cardiac, cerebrovascular, or cardiac valve disease, 5% had inflammatory bowel disease or rheumatic disease, 2% had moderate to severe renal disease, 2% had moderate to severe liver disease, 4% had an infection requiring ongoing antimicrobial treatment, and 13% had a prior malignancy other than non-melanoma skin cancer. 18 patients had previously undergone allogeneic hematopoietic cell transplantation (HCT), and 35 patients had previously received checkpoint inhibitor therapy.
Efficacy
With a median follow-up of 12.9 months, the best overall response rate (ORR) for the 1,297 patients was 73%, including 56% CR and 18% partial response (PR). The median duration of response (DOR) was not reached, with 64% and 57% of patients remaining relapse/progression-free at 12 and 24 months after initial response, respectively. The Kaplan-Meier (KM) estimates for DOR are shown in Figure 1A.
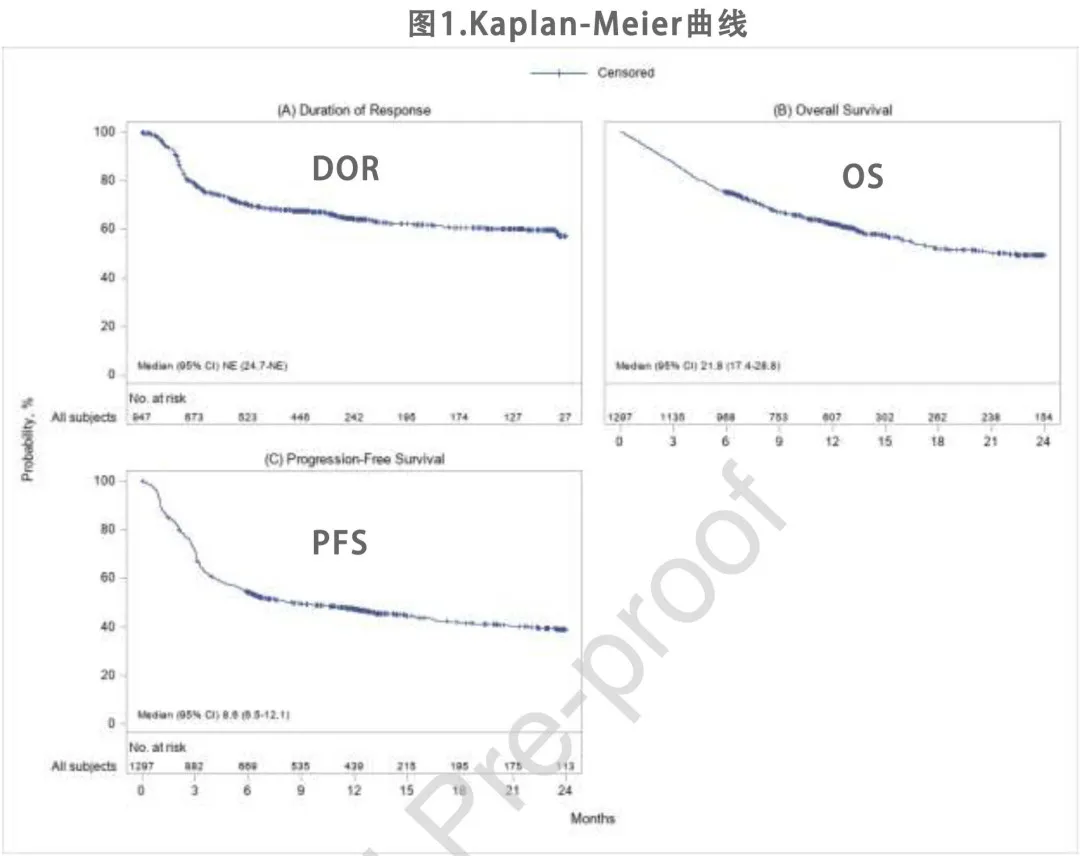
The KM estimates for OS and progression-free survival (PFS) are shown in Figures 1B and 1C. The median OS was 21.8 months, with an estimated 62% and 50% of patients alive at 12 and 24 months after infusion, respectively. The median PFS was 8.6 months, with an estimated 47% and 39% of patients being relapse/progression-free and alive at 12 and 24 months, respectively. At the time of data cutoff, 544 deaths (42%) were reported, with disease relapse or progression being the most common cause of death (74% of deceased patients), followed by infection (11%) and organ failure (5%). The non-relapse mortality (NRM) rate was 1% at 1 month and 3% at 3 months (Table 2).
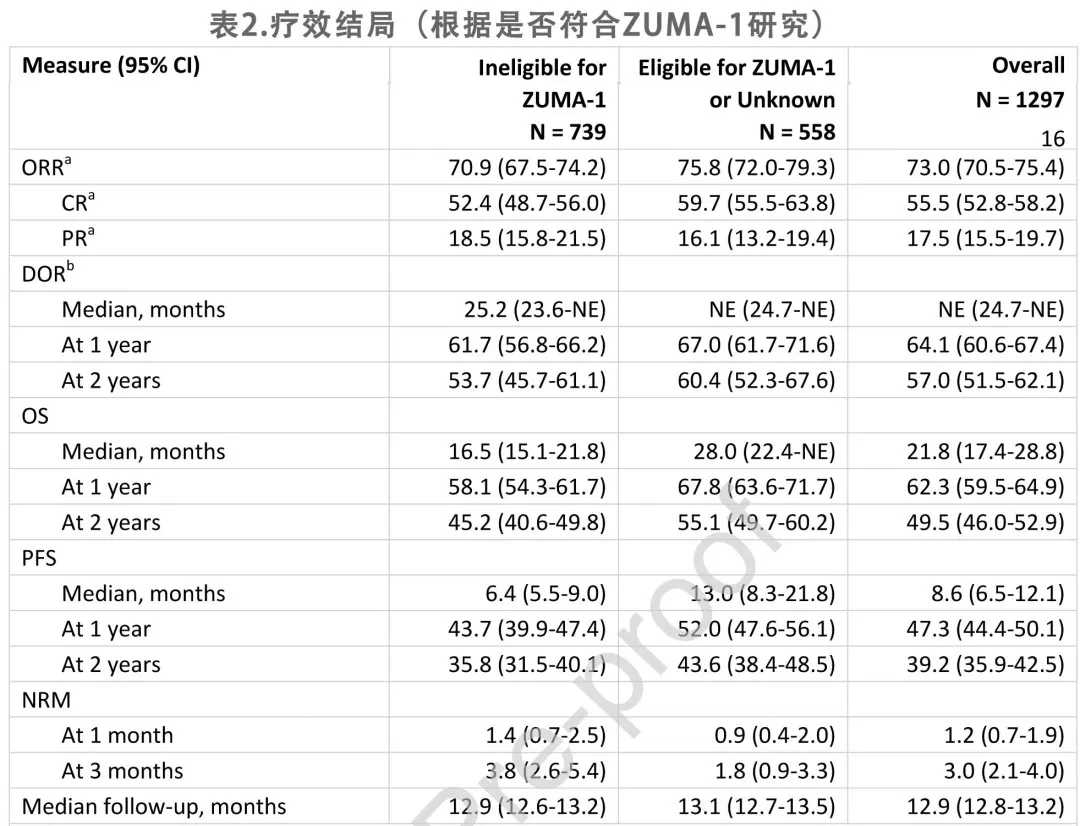
Based on multivariable analyses, pre-infusion ECOG performance status ≥2 and chemorefractory disease were associated with lower ORR (odds ratio [OR] = 0.32 and 0.54, respectively), shorter DOR (hazard ratio [HR] = 3.29 and 1.39, respectively), shorter OS (HR = 3.27 and 1.44, respectively), and shorter PFS (HR = 2.61 and 1.48, respectively). Moderate to severe lung disease was associated with lower ORR (OR = 0.75), while moderate to severe liver disease was associated with shorter DOR (HR = 2.63), OS (HR = 2.69), and PFS (HR = 2.38). Additionally, patients aged ≥65 years had a higher ORR (OR = 1.39).
Safety
As shown in Table 3, among the 1,297 patients, 1,073 (83%) experienced any-grade cytokine release syndrome (CRS), and 107 (8%) experienced grade ≥3 CRS; 714 (55%) experienced any-grade immune effector cell-associated neurotoxicity syndrome (ICANS), with 313 (24%) being grade ≥3. The median time to onset of CRS and ICANS from axi-cel infusion was 4 days and 7 days, respectively.
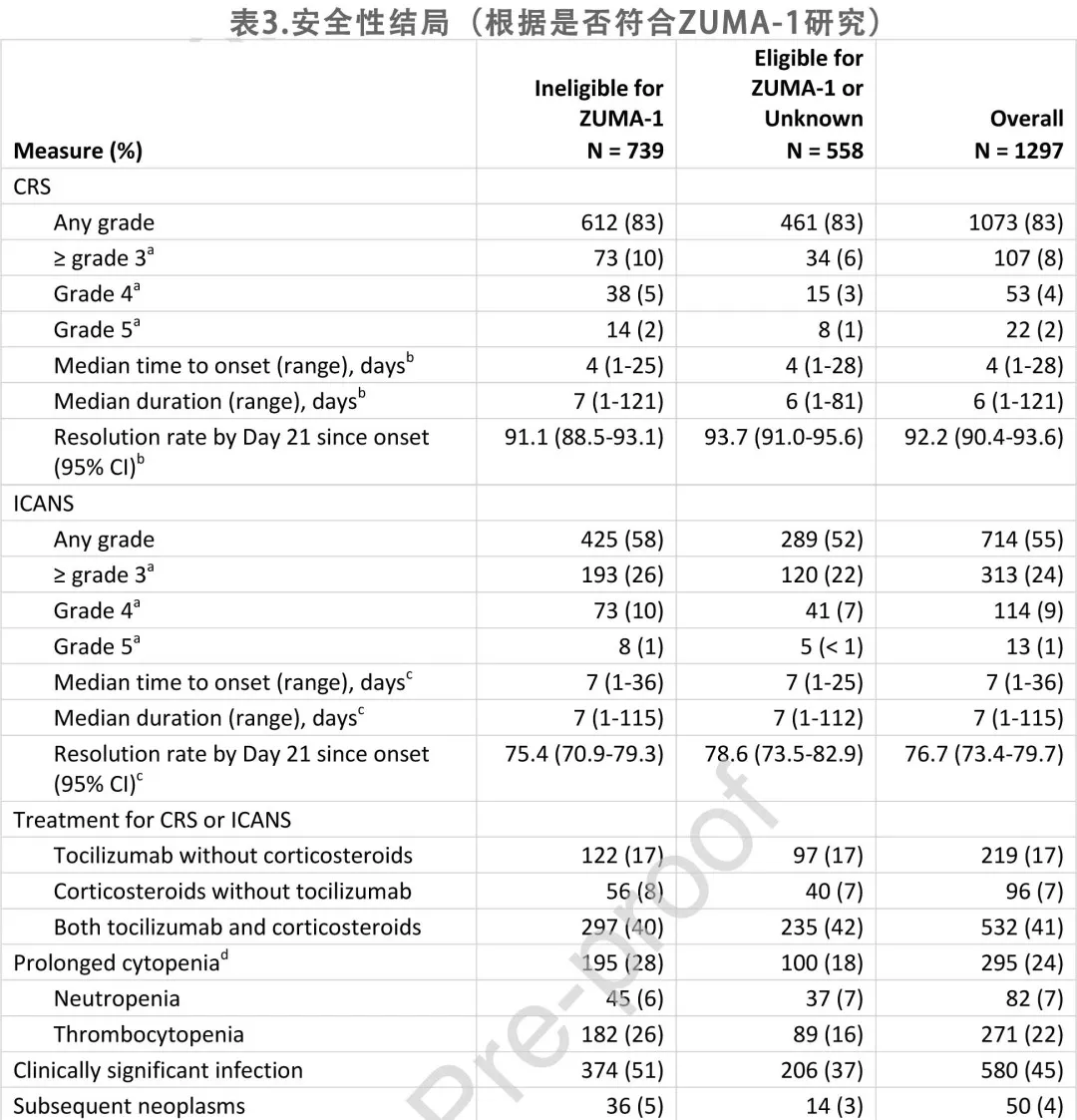
219 patients (17%) received tocilizumab without corticosteroids, 96 (7%) received corticosteroids but not tocilizumab, and 532 (68%) received both; 79% of patients with concurrent CRS and ICANS received tocilizumab, while 53% of patients with CRS alone received tocilizumab. Additionally, 81% of patients with concurrent CRS and ICANS received corticosteroids. The resolution rates for CRS and ICANS by week 3 after onset were 92% and 77%, respectively.
At the time of data cutoff, 295 patients (24% of those alive at 30 days post-infusion) experienced prolonged cytopenias, including 82 (7%) with neutropenia and 271 (22%) with thrombocytopenia; 580 patients (45%) experienced clinically significant infections. There were 50 patients (4%) with secondary malignancies, with myelodysplastic syndrome (n = 15), squamous cell carcinoma of the skin (n = 11), and myelodysplastic/myeloproliferative neoplasm (n = 4) being the most common; 2 patients had multiple secondary malignancies.
Based on multivariable analyses, age ≥65 years was associated with an increased risk of CRS (OR = 1.41) and ICANS (OR = 1.77). Moderate to severe liver disease was associated with a higher odds of grade ≥3 CRS (OR = 3.70), while ECOG performance status ≥2 was associated with a higher incidence of ICANS (OR = 2.63) and grade ≥3 ICANS (OR = 3.23).
Discussion
Based on data from 1,297 patients across 78 centers, this study represents the largest prospective real-world study of patients receiving CAR-T cell therapy to date. The results indicate that the efficacy of axi-cel for the treatment of r/r LBCL in the real-world setting (ORR 73%, CR 56%) is comparable to the pivotal ZUMA-1 clinical trial.
The study also demonstrates that patients who did not meet the eligibility criteria for the ZUMA-1 trial had a comparable DOR (62% at 1 year) to the ZUMA-1 eligible cohort (67% at 1 year). Furthermore, certain comorbidities, such as prior malignancies, which were exclusion criteria in the ZUMA-1 study, were not associated with inferior efficacy outcomes. Notably, even after multivariable adjustment, patients aged ≥65 years had a 39% higher odds of achieving an overall response compared to younger patients. Additionally, the non-relapse mortality (NRM) rate following axi-cel infusion remained remarkably low.
The incidence of CRS in this study was similar to that reported in the ZUMA-1 trial, but there was a trend towards a lower rate of grade ≥3 CRS (8% vs. 11% in ZUMA-1). The median time to CRS onset was 4 days, and the median time to CRS resolution was 6 days, compared to 2 days and 8 days, respectively, in ZUMA-1. Similarly, the incidence of grade ≥3 ICANS was 24% in this study, compared to 28% in ZUMA-1. The median time to ICANS onset was 7 days, whereas it was 5 days in ZUMA-1. Apart from moderate to severe liver disease being associated with grade ≥3 CRS, the other assessed comorbidities were not associated with CRS or ICANS. Compared to the pivotal axi-cel clinical trial, the incidence of thrombocytopenia, neutropenia, and infections was also lower. The improved safety profile of grade ≥3 CRS and ICANS in the real-world setting compared to early clinical trials may be primarily due to the increased use of tocilizumab, corticosteroids, siltuximab, and other agents as part of the new toxicity management paradigm for CAR-T therapy, while lower pre-treatment inflammatory levels and disease burden owing to improved bridging therapy may explain the longer median times to CRS and ICANS onset.
In summary, the study results demonstrate that patients who did not meet the eligibility criteria for the pivotal ZUMA-1 trial still achieved durable responses with axi-cel. Despite the high incidence of CRS and ICANS, elderly patients had favorable efficacy outcomes with axi-cel. Patient selection for standard axi-cel therapy should consider comorbidities and the risk-benefit ratio, rather than strictly adhering to the ZUMA-1 eligibility criteria. The study findings substantially increase the evidence for the use of axi-cel in the real-world setting and expand the definition of the potential patient population that may benefit from it.
References
Caron A Jacobson,et al.Real-world Evidence of Axicabtagene Ciloleucel for the Treatment of Large B-Cell Lymphoma in the United States.Transplant Cell Ther . 2022 May 21;S2666-6367(22)01319-7. doi: 10.1016/j.jtct.2022.05.026.
Content Source:聊聊血液
