July 24, 2020 Brexucabtagene Autoleucel FDA Approval for the Treatment of Relapsed / Refractory Mantle Cell Lymphoma (MCL)
July 24, 2020 Brexucabtagene Autoleucel FDA Approval for the Treatment of Relapsed / Refractory Mantle Cell Lymphoma (MCL)
On July 24, 2020, the U.S. FDA announced accelerated approval of the CAR-T cell therapy Brexucabtagene Autoleucel (Tecartus) for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
This is the first CAR-T therapy approved for MCL and the third CAR-T therapy approved globally. Previously, Brexucabtagene Autoleucel had received “Priority Review” and “Breakthrough Therapy” designations.
Mantle Cell Lymphoma (MCL)
MCL is a relatively rare subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 6% of all NHL cases. It originates from cells in the “mantle zone” of lymph nodes, hence its name, and is more common in middle-aged and older adults.
In MCL patients, B cells (a type of immune cell) transform into cancer cells, initially forming tumors in the lymph nodes and then rapidly spreading to other parts of the body. This lymphoma combines the aggressive progression of invasive lymphomas and the difficulty in curing indolent lymphomas.
Currently, MCL is primarily treated with chemotherapy and other drug combinations. For younger patients with early relapse after initial treatment, stem cell transplantation may also be an option. However, once the disease becomes resistant to existing therapies, clinical treatment options become limited. Additionally, most MCL patients are diagnosed at an advanced stage with aggressive disease, making it difficult to cure and resulting in low 5-year survival rates. Therefore, MCL patients urgently need new treatment options.
CAR-T Cell Therapy Brexucabtagene Autoleucel
The basic principle of CAR-T cell immunotherapy is to use genetic engineering to modify T lymphocytes to express chimeric antigen receptors, enabling them to kill tumor cells.
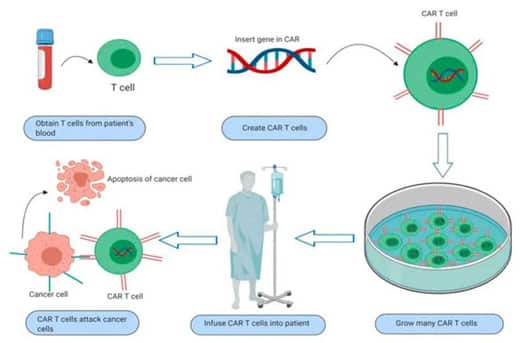
Brexucabtagene Autoleucel is an autologous CAR-T cell therapy targeting CD19. It involves collecting the patient’s peripheral blood and extracting T cells, which are then genetically modified ex vivo to equip them with the ability to recognize tumor cell surface antigens.
These modified T cells are then infused back into the patient, precisely identifying and killing tumor cells while avoiding damage to normal tissues. It can be considered a “living drug.”
Previously approved CAR-T cell immunotherapies have primarily been used to treat B-cell acute lymphoblastic leukemia (B-ALL), chronic lymphocytic leukemia, and B-cell non-Hodgkin lymphoma. In recent years, CAR-T therapy has also been used in experimental treatments for solid tumors and HIV, among others.
Due to its significant efficacy in hematological malignancies, CAR-T therapy has become one of the most promising anti-cancer therapies globally.
Clinical Trial
The approval of Brexucabtagene Autoleucel was based on the results of the pivotal ZUMA-2 clinical trial, which enrolled 74 adult patients with relapsed or refractory MCL who had previously received chemotherapy, anti-CD20 antibody therapy, and BTK inhibitor therapy, with a follow-up period of over 6 months.
The results showed that among the 60 patients evaluable for efficacy, Brexucabtagene Autoleucel treatment achieved a complete response rate of 62% and an overall response rate of 87%.
Currently, Brexucabtagene Autoleucel is being investigated in phase 1/2 clinical trials for the treatment of acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and MCL.
