Breyanzi Second Line is Effective in the Treatment of Relapsed or Refractory Large B-cell Lymphoma (r/r lbcl)
Breyanzi Second Line is Effective in the Treatment of Relapsed or Refractory Large B-cell Lymphoma (r/r lbcl)
In January 2022, Bristol Myers Squibb (BMS) announced the pre-specified interim analysis data from the pivotal Phase 3 TRANSFORM study (NCT03575351). This is a global, multicenter, randomized Phase 3 study evaluating the efficacy and safety of the CD19 CAR-T cell therapy Breyanzi (lisocabtagene maraleucel, liso-cel) as a second-line treatment for adult patients with relapsed or refractory large B-cell lymphoma (R/R LBCL). The interim analysis showed that the study met its primary and key secondary endpoints: In patients with relapsed or refractory (R/R) LBCL who are eligible for hematopoietic stem cell transplantation (HSCT), Breyanzi demonstrated significantly improved efficacy and a favorable safety profile compared to the current standard of care (salvage chemotherapy followed by high-dose chemotherapy [HDCT] and HSCT).
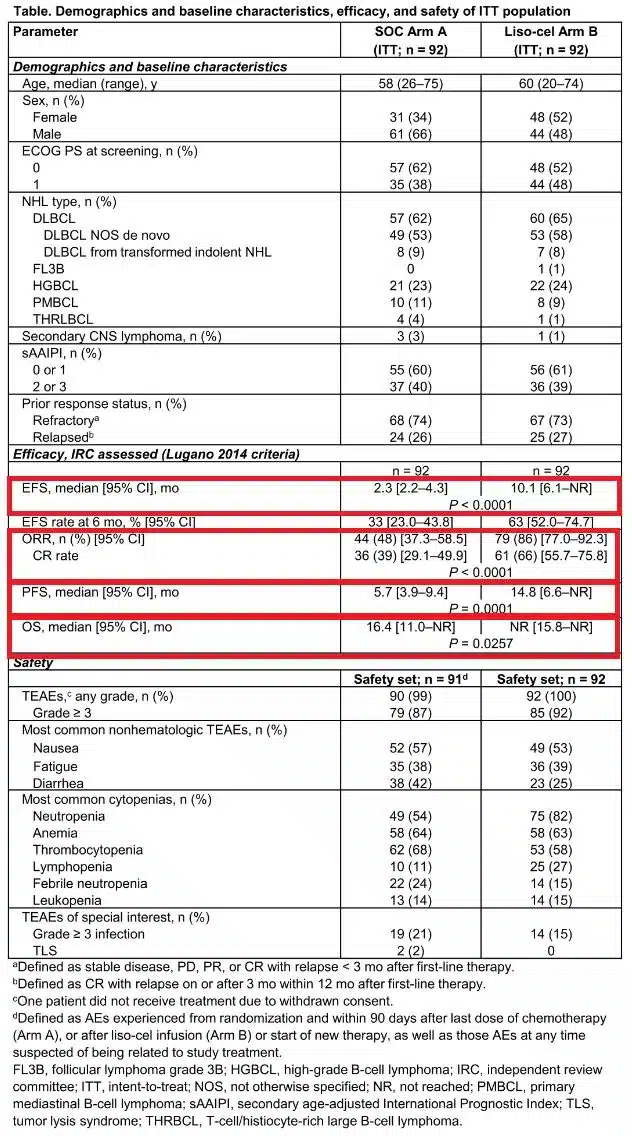
Specifically, with a median follow-up of 6.2 months, Breyanzi significantly prolonged event-free survival (EFS, the study’s primary endpoint) compared to standard of care: The median EFS was 10.1 months (95% CI: 6.1-NR) in the Breyanzi arm versus 2.3 months (95% CI: 2.2-4.3) in the standard of care arm. Breyanzi reduced the risk of EFS events by 65% compared to standard of care (HR=0.349; p<0.0001).
Breyanzi is an autologous, CD19-directed, chimeric antigen receptor (CAR) T-cell therapy with a defined composition and a 4-1BB costimulatory domain. Breyanzi is composed of purified CD8+ and CD4+ T cells in a specific ratio (1:1), and the 4-1BB signaling enhances the expansion and persistence of Breyanzi.
In February 2020, Breyanzi received FDA approval for the treatment of adult patients with R/R LBCL, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B, who have received at least two prior systemic therapies. Breyanzi is not indicated for the treatment of patients with primary central nervous system lymphoma.
The results from the TRANSFORM study demonstrate for the first time that in patients with relapsed or refractory LBCL, a treatment approach is more effective than the standard high-dose chemotherapy and stem cell transplantation, and provide the first evidence of the potential of CD19-directed CAR-T cell therapy as a second-line treatment.
Manali Kamdar, M.D., Director of the Hematologic Malignancies and Stem Cell Transplant Program at the University of Colorado Cancer Center, commented: “For over 20 years, salvage chemotherapy followed by high-dose chemotherapy and stem cell transplant has been the standard of care for second-line treatment of patients with relapsed or refractory LBCL, but only a small portion of patients derive long-term benefit from this approach. In the Phase 3 TRANSFORM study, Breyanzi outperformed the current standard of care for patients with difficult-to-treat disease, and these results have the potential to pave the way for a treatment paradigm shift where patients with disease that has relapsed or is refractory to front-line therapy can be treated with a personalized CAR-T cell therapy, potentially improving treatment outcomes.”
Interim Analysis Results from the TRANSFORM Study
TRANSFORM is a global, randomized, multicenter study conducted in adult patients with primary refractory or relapsed large B-cell lymphoma (LBCL) within 12 months of first-line therapy, who are eligible for autologous stem cell transplantation (ASCT), evaluating the efficacy and safety of the CD19 CAR-T cell therapy Breyanzi (lisocabtagene maraleucel, liso-cel) as a second-line treatment compared to the current standard of care (including high-dose chemotherapy [HDCT] and ASCT).
In this study, 184 patients with primary refractory LBCL or relapsed disease within ≤12 months of first-line therapy and eligible for ASCT were randomized to receive Breyanzi (n=92) or salvage chemotherapy followed by HDCT and ASCT (n=92), which is considered the current standard of care for these patients. In this crossover-permitted trial, 50 patients from the standard of care arm crossed over to receive Breyanzi after failing to achieve a response by week 9 (after three cycles of salvage chemotherapy) or at any time after disease progression.
The data showed that the majority of patients (86%) receiving Breyanzi achieved a complete or partial response, with 66% achieving a complete response (CR). In contrast, less than half (48%) of patients receiving standard of care achieved a response, with only 39% achieving a CR (p<0.0001). Compared to standard of care, Breyanzi treatment resulted in a significantly longer median progression-free survival (PFS) (14.8 months vs. 5.7 months [HR=0.406; p=0.0001]). Although overall survival (OS) data were immature, the pre-specified interim analysis showed a strong trend favoring Breyanzi over standard of care (HR=0.509, 95% CI: 0.258-1.004, p=0.0257).
In the study, Breyanzi demonstrated a manageable safety profile, with very low incidences of severe cytokine release syndrome (CRS) and neurological events (NE), and no new safety signals were observed in the second-line treatment setting. No Grade 4/5 CRS or NE were reported in the study. Forty-nine percent of patients reported any-grade CRS, with only one patient reporting Grade 3 CRS. Among patients receiving Breyanzi, 12% reported any-grade NE, and four patients (4%) reported Grade 3 NE.
Content Source:生物谷
