CAR-T Cell Kymriah (Tisagenlecleucel) Demonstrates Durable Responses and Favorable Safety in Patients with Relapsed/Refractory Follicular Lymphoma
CAR-T Cell Kymriah (Tisagenlecleucel) Demonstrates Durable Responses and Favorable Safety in Patients with Relapsed/Refractory Follicular Lymphoma
Follicular lymphoma (FL), an indolent form of non-Hodgkin lymphoma, is characterized by a relapsing and remitting course, making treatment exceptionally complex. For patients with relapsed or refractory (R/R) FL, there is currently no clear standard of care (SOC). Although immunochemotherapy is initially effective, its efficacy gradually diminishes, and toxicity may accumulate. However, the advent of Kymriah (tisagenlecleucel) has broken this impasse, as it is approved in the United States, European Union, and Japan for third-line treatment of R/R FL, demonstrating promising efficacy in clinical trials. Recently, German scholars published a clinical study in the prestigious journal Blood, aiming to evaluate the safety and efficacy of Kymriah in patients with R/R FL. The results showed that Kymriah exhibited significant and durable therapeutic effects, with favorable safety.
Background: Kymriah Breaks Treatment Impasse and Brings Hope for R/R FL
FL is considered an indolent non-Hodgkin lymphoma characterized by a relapsing and remitting course. For patients with R/R FL, there is currently no clear standard of care (SOC), and immunochemotherapy is repeatedly used from the initial treatment to subsequent treatment stages, but its efficacy gradually diminishes, and toxicity may accumulate. Patients with high-risk disease, such as those who experience disease progression within 24 months after initial immunochemotherapy (POD24) and those with a high baseline tumor burden, have a poor prognosis and an increased risk of death.
In the primary analysis of the single-arm, open-label, phase 2 ELARA trial, Kymriah demonstrated a high overall response rate (ORR; 86%), complete response rate (CRR; 69%), and durable responses (12-month progression-free survival [PFS] of 67%) in adult patients with high-risk R/R FL. ≤1% of patients experienced grade ≥3 cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. This report, based on the follow-up data of more than two years after Kymriah treatment in the ELARA trial, introduces the durability of sustained responses, long-term safety, as well as relevant biomarker and pharmacokinetic analyses.
Key Findings: Kymriah Demonstrates Significant Long-Term Efficacy in Patients with High-Risk Follicular Lymphoma, and Two-Year Follow-Up Data Reveal Durable Responses and Safety
As of March 29, 2022, a total of 97 patients received Kymriah cell therapy. The median time from the first-line treatment to enrollment in the ELARA trial was 59.5 months. The median follow-up time after Kymriah infusion was 28.9 months. The efficacy analysis included 94 patients with evaluable disease conditions at the time of infusion, while the safety analysis included all 97 patients who received cell therapy. Baseline characteristics showed that the average number of treatment lines was 4, and 35 patients (36%) had previously undergone autologous hematopoietic stem cell transplantation. Of the 97 patients, 76 (78%) were refractory to previous treatments, 61 (63%) experienced disease progression within 2 years after initial anti-CD20 treatment (POD24 group), 58 (60%) had a FLIPI score ≥3, 44 patients (45%) received bridging therapy, and 18% of patients received Kymriah as outpatients.
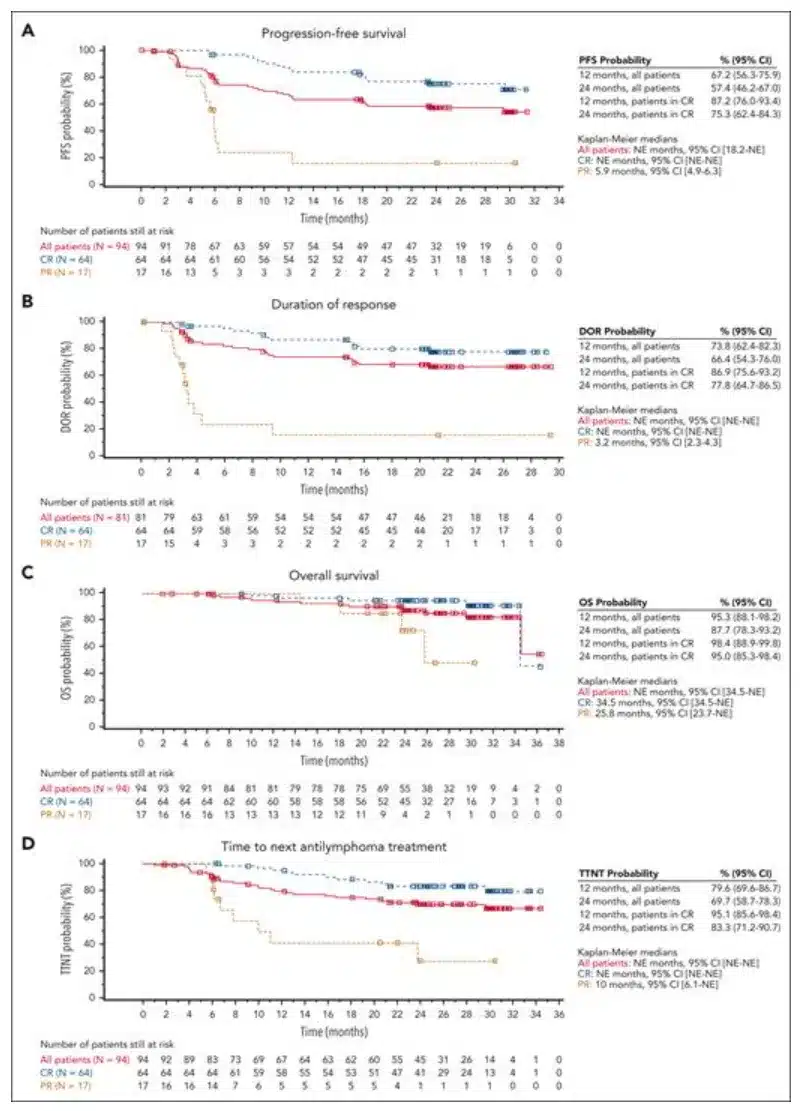
Figure 1: Kaplan-Meier Curves for Key Endpoints in Patients with R/R FL Who Received Kymriah Infusion
In this updated follow-up, the median progression-free survival (PFS), median duration of response (DOR), median overall survival (OS), and median time to next treatment (TTNT) were not reached. Patients who achieved CR performed better than the overall population on all efficacy endpoints. Twenty-five (31%) of the Kymriah responders experienced relapse, with a median time to relapse of 121.5 days. The overall response rate (ORR) was 86.2%, and the complete response rate (CRR) was 68.1%. Among the 31 patients who initially achieved partial remission, 14 converted to CR within 6 months. Patients with high-risk baseline characteristics also showed high rates of durable responses. The ORR for POD24 patients was 82%, and the CRR was 59%; for patients with high tumor burden, the ORR was 75%, and the CRR was 40%; for patients with high FLIPI scores, the ORR was 80.7%, and the CRR was 61.4%.
The safety profile of Kymriah was consistent with previous reports, with no new safety events or treatment-related deaths. Sixteen patients (16.5%) experienced infections after infusion, including 9 cases (9.3%) of serious infections. By month 6, the resolution rate for various types of cytopenias was 82.0%, and by month 24, it was 96.7%. Two patients experienced serious neurological events 8 weeks after infusion.
Researchers’ Comments
The long-term updated results from the ELARA trial continue to confirm that patients with R/R FL treated with Kymriah achieve high response rates and durable responses, with a favorable safety profile. After more than 2 years of follow-up, the median DOR, PFS, OS, and TTNT have not been reached.
Durable anti-tumor effects were observed in most patients, including those with high-risk clinical features. The long-term efficacy in these patients supports the use of Kymriah in a broad range of R/R FL patients.
As more treatment options become available for patients with R/R FL, the question of how to individualize treatment to achieve optimal outcomes remains, and further long-term follow-up and real-world data are needed.
Overall, the more than 2-year long-term follow-up from ELARA demonstrates that Kymriah shows significant and durable efficacy, with favorable safety, in adult patients with R/R FL, including high-risk patients who currently lack effective treatment options.
Content Source:血液肿瘤资讯
