CAR-T Cell Treatment for Lymphoma, Igniting New Hope for Patients’ Survival
CAR-T Cell Treatment for Lymphoma, Igniting New Hope for Patients’ Survival
June 22nd, CAR-T Day: CAR-T Ignites New Hope for Patients with Relapsed/Refractory Lymphoma
June 22nd is designated as CAR-T Day, to commemorate the official approval and launch of Axicabtagene Ciloleucel in China, marking the beginning of the CAR-T cell therapy era in China.
Today, let’s explore how CAR-T brings the miracle of cure to patients with lymphoma!
Unveiling the Mystery of Relapsed/Refractory Lymphoma
Ding! This briefing tells you about relapsed/refractory lymphoma. Diffuse large B-cell lymphoma (DLBCL) is a highly heterogeneous malignancy of the hematopoietic system[1]. Approximately 40% of DLBCL patients experience relapse or develop refractory disease after first-line treatment, progressing to relapsed or refractory DLBCL (R/R DLBCL)[2]. Among R/R DLBCL patients, less than half benefit from second-line salvage immunochemotherapy, and the prognosis for third-line treatment is poor[2]. What if it relapses or becomes refractory? Don’t worry, there’s a solution here. The advent of autologous chimeric antigen receptor T (CAR-T) cell therapy has brought new hope for the cure of R/R DLBCL patients[3].
Unraveling the Mysteries of CAR-T Cell Therapy
CAR-T cell therapy is a major breakthrough in the treatment of lymphoma in recent years and is a form of immunotherapy. The principle of CAR-T therapy is to activate the body’s immune “warriors,” T cells, through genetic engineering techniques and install a targeting navigation device called CAR (chimeric antigen receptor). This transforms the ordinary “soldier” T cells into “super soldiers,” known as “CAR-T cells.” They can release various effector factors through immune actions, efficiently killing tumor cells, thereby achieving the treatment of malignant tumors. If natural T cells are considered “regular troops,” then CAR-T cells are akin to “special forces”[4]. CAR-T cell therapy primarily involves three steps: first, collecting the patient’s T cells; second, modifying the T cells to become CAR-T cells; and third, infusing the CAR-T cells back into the patient’s body to kill the cancer cells[1].
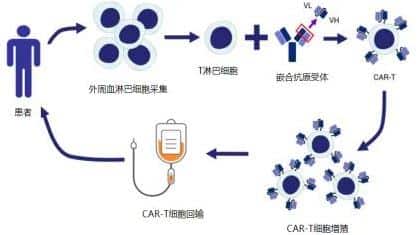
Witnessing the Miraculous Power of CAR-T Cell Therapy
CAR-T cell therapy targeting CD19 has demonstrated significant clinical benefits and has advanced the treatment of relapsed DLBCL since its first report[5]. Axicabtagene ciloleucel (Axi-cel) is a single-infusion, CD19-directed CAR-T cell therapy[6]. It has now been approved for marketing[7] for the treatment of adult patients with R/R DLBCL after two or more lines of systemic therapy. Axi-cel achieved an objective response rate (ORR) of 83% in the third-line treatment of R/R DLBCL, with more than half of patients achieving complete remission[9]. Compared to traditional chemotherapy, Axi-cel can reduce the risk of death in patients with relapsed or refractory lymphoma by 73%[10]. In the ZUMA-1 study, after following up on Axi-cel-treated R/R DLBCL patients for more than 5 years, the exciting news is that their 5-year survival rate reached 42.6%! Even more remarkably, those patients who achieved complete remission within three months had a 5-year survival rate of 64.4%[5]! This prompts us to speculate: Have these patients conquered the disease and achieved true cure?
CAR-T Cell Therapy Brings Hope, Igniting New Possibilities
Axi-cel has demonstrated sustained survival benefits in R/R DLBCL patients with good safety. More importantly, it brings the potential for cure to most patients with relapsed or refractory lymphoma, igniting new hope for them. Relapsed or refractory lymphoma is not an incurable disease! Maintain a positive attitude, and let CAR-T cell therapy create hope alongside you, together conquering relapsed or refractory lymphoma.
References:
1. Thieblemont C, et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol. 2023 Apr 20;41(12):2238-2247.
2. Iacoboni G, et al. Real-world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B-cell lymphoma. Cancer Med. 2021 May;10(10):3214-3223.
3. Houot R, et al. Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: a phase 2 trial. Nat Med. 2023 Oct;29(10):2593-2601.
4. Zhao Z, et al. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018 Jul;8(4):539-551.
5. Neelapu SS,et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. 2023 May 11;141(19):2307-2315.
6. Westin JR, et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N Engl J Med. 2023 Jul 13;389(2):148-157.
7. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20220601110541120.html
8. 阿基仑赛注射液说明书(更新于2023年6月21日)
9. Locke FL, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019 Jan;20(1):31-42.
10. Neelapu SS, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021 Oct 26;5(20):4149-4155.
Content Source:战胜086
