Phase 2 Clinical Study of Lisocabtagene Maraleucel (liso-cel) CAR-T Follicular Lymphoma with More Than 2 Lines of Treatment
Phase 2 Clinical Study of Lisocabtagene Maraleucel (liso-cel) CAR-T Follicular Lymphoma with More Than 2 Lines of Treatment
CAR-T Therapy for Follicular Lymphoma
For patients with relapsed/refractory (R/R) follicular lymphoma (FL) and high-risk disease features (e.g., progression of disease within 24 months after first-line immunochemotherapy [POD24] and double refractory to anti-CD20 agents and alkylating agents), there remains an unmet need due to the lack of established standard treatments and poor prognosis. CAR-T cell therapy is an effective choice for patients with R/R FL after ≥2 prior lines of systemic treatment, but the optimal timing for CAR-T therapy during the FL disease course is not yet established, and there is a lack of data for its use in second-line (2L) treatment of patients with high-risk features.
Lisocabtagene maraleucel (liso-cel) is an autologous CD19 4-1BB CAR-T cell product that has demonstrated high response rates and deep responses in treating R/R B-cell lymphomas. The pivotal phase 2 TRANSCEND study evaluated the efficacy and safety of liso-cel in treating patients with R/R indolent non-Hodgkin’s lymphoma, and recently reported in Nature Medicine were the preliminary results from the R/R FL cohort with ≥1 prior line of treatment, including the first report of CAR-T therapy in second-line R/R FL.

Study Results
The FL cohort of the phase 2 TRANSCEND study enrolled patients with R/R FL after ≥1 prior line of treatment who underwent leukapheresis. The 2L FL cohort had received one prior line of treatment and met criteria for POD24 (defined as anti-CD20 antibody and alkylating agent treatment within 6 months of diagnosis and disease progression within 24 months) and/or ≥1 modified GELF (mGELF) criteria. A total of 139 patients with 2L+ FL were enrolled, including 114 with 3L+ and 25 with high-risk 2L disease. Successful liso-cel manufacturing occurred in 133 patients (96%), with 4 patients not receiving liso-cel and 5 receiving non-conforming product (Figure 1).
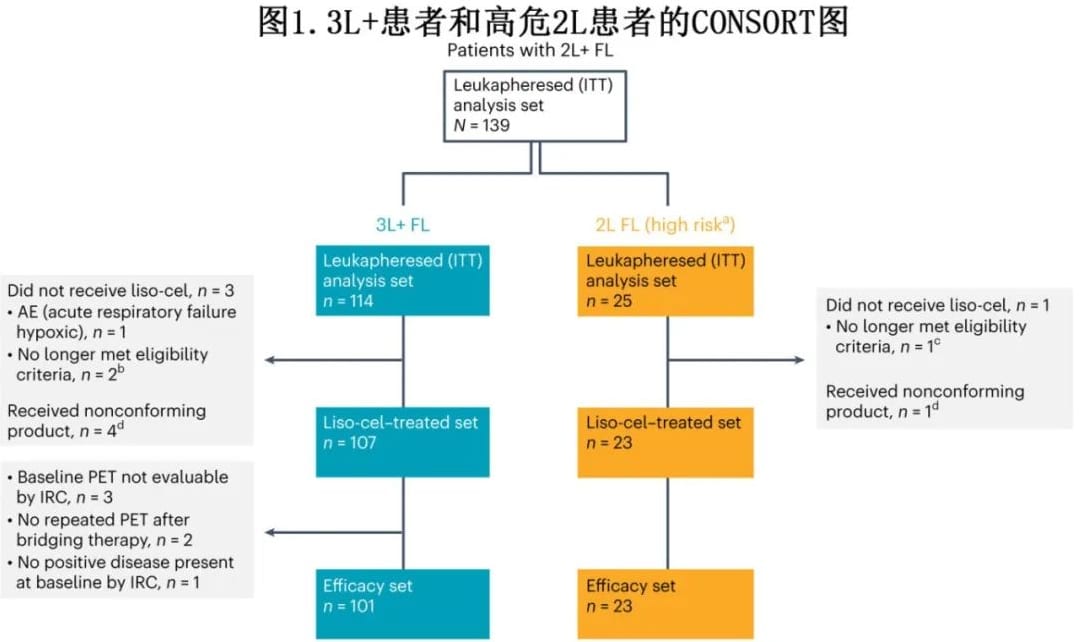
A total of 130 patients with 2L+ FL (2L, n=23; 3L+, n=107) received liso-cel treatment (liso-cel treated set), and 124 patients (2L, n=23; 3L+, n=101) were evaluable for efficacy by an independent review committee (IRC) (efficacy set). In the liso-cel treated set, the median time from leukapheresis to liso-cel availability was 29 days, and from leukapheresis to liso-cel infusion was 49 days. At data cutoff, the median follow-up time was 18.9 months.
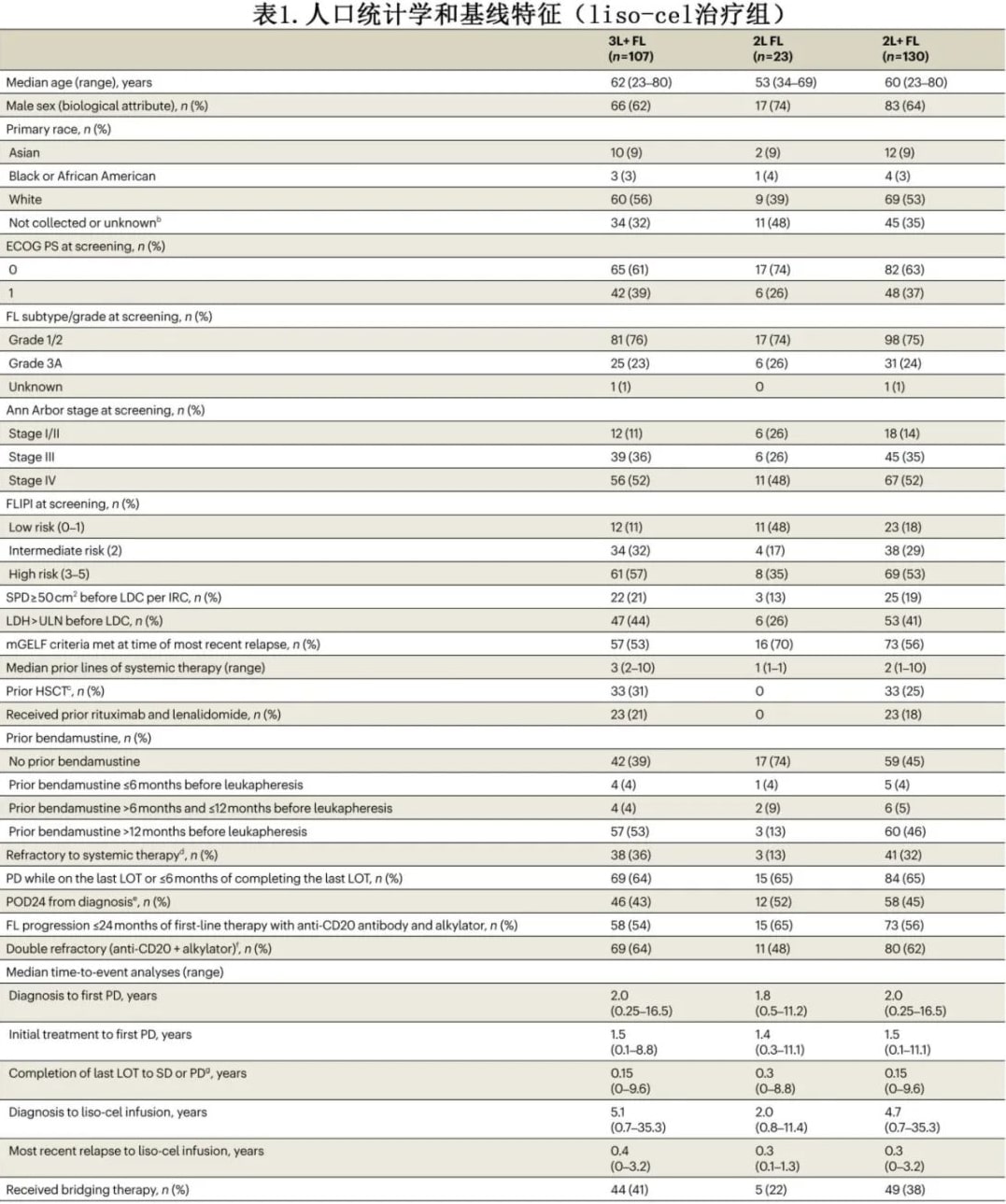
In the liso-cel treated set, the median age was 60 years (3L+, 62 years; 2L, 53 years); 86% had Ann Arbor stage III/IV disease (3L+, 89%; 2L, 74%); 53% had high-risk FLIPI (3L+, 57%; 2L, 35%); 45% had POD24 (3L+, 43%; 2L, 52%); 56% met mGELF criteria (3L+, 53%; 2L, 70%); and 62% were double refractory (3L+, 64%; 2L, 48%) (Table 1). Fifty-six percent of patients had POD24 after first-line anti-CD20 antibody plus alkylating agent systemic therapy (3L+, 54%; 2L, 65%). The median time from diagnosis to liso-cel infusion was 4.7 years (3L+, 5.1 years; 2L, 2.0 years). Thirty-eight percent of patients received bridging therapy to control disease during liso-cel manufacturing (3L+, 41%; 2L, 22%) (Table 1). Most bridging therapies were combination regimens, primarily rituximab plus gemcitabine and oxaliplatin.
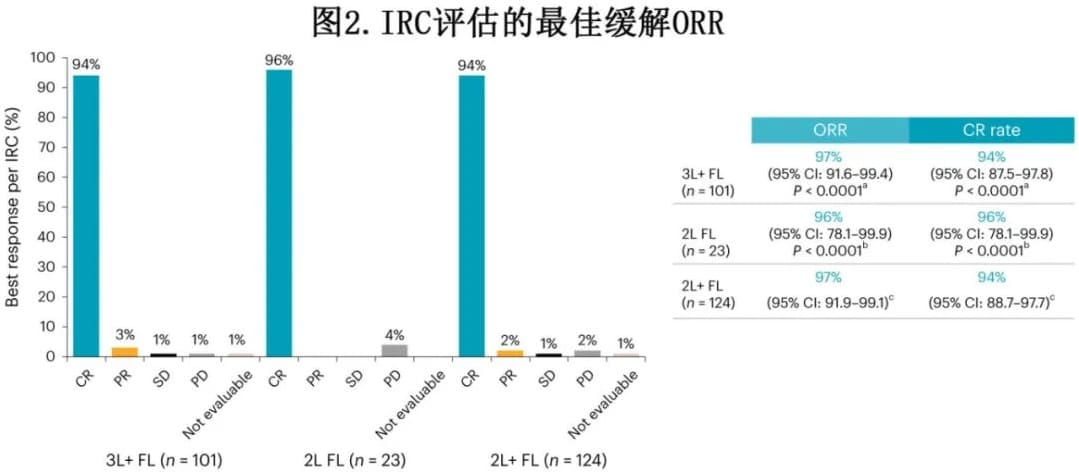
Efficacy
In the 3L+ FL cohort, the overall response rate (ORR) was 97%, with a 94% complete response (CR) rate, and the median time to first response was 1 month. In the 2L FL cohort, the ORR was 96%, all of which were CRs, with a median time to first response of 1 month (Figure 2).
In the 3L+ FL cohort, with a median follow-up of 16.6 months, the median duration of response (DOR) was not reached, and the 12-month DOR rate was 82% (Figure 3). With a median follow-up of 17.6 months, the median progression-free survival (PFS) was not reached, and the 12-month PFS rate was 81%. The median overall survival (OS) was not reached, with a 12-month OS rate of 92%. In the 2L FL cohort, with a median follow-up of 16.8 months, the median DOR was not reached, and the 12-month DOR rate was 90%. With a median follow-up of 17.8 months, the median PFS was not reached, and the 12-month PFS rate was 91%. The median OS was not reached, with a 12-month OS rate of 96%.
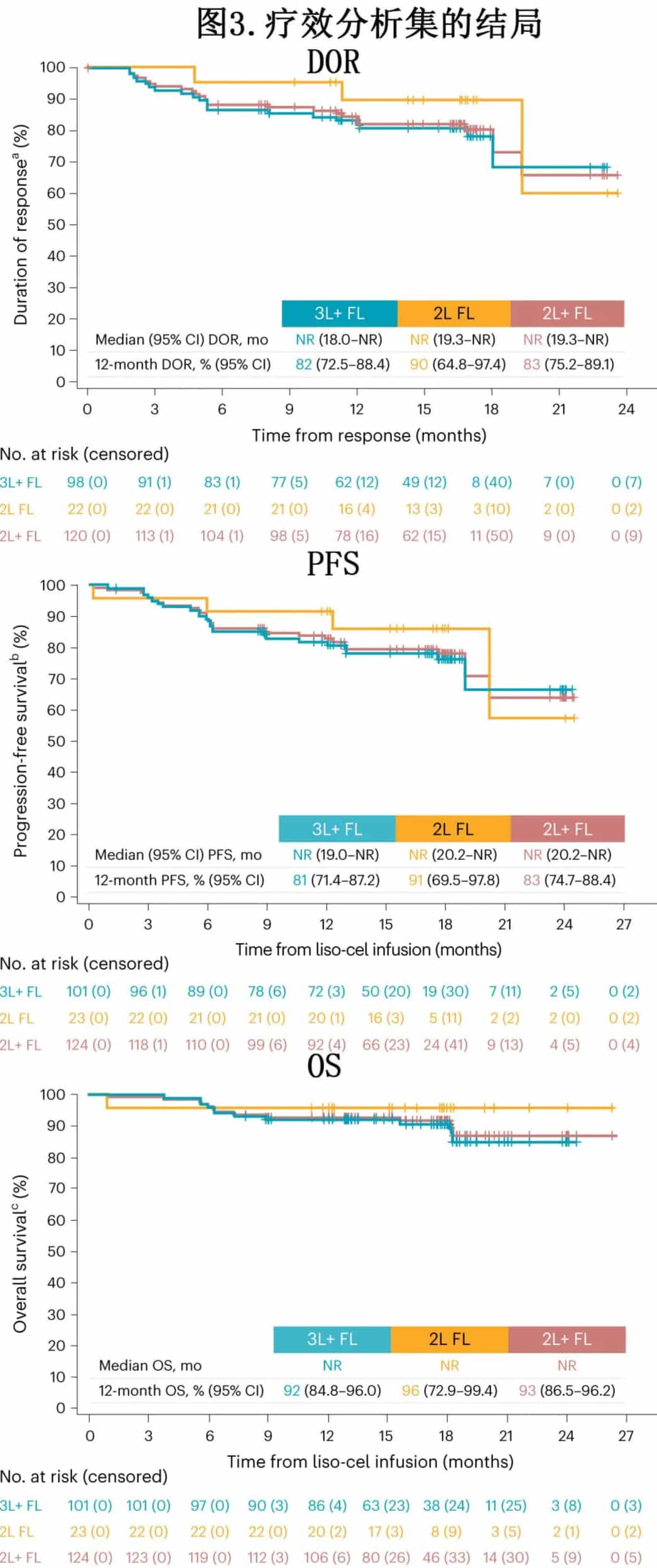
Subgroup analyses in the efficacy set were consistent with the initial analyses, demonstrating high ORR, CR rates, DOR, and PFS, including in patients with high-risk disease features.
Response rates in the intention-to-treat (ITT) (leukapheresis) population were similar, with an ORR of 93% and CR rate of 90% in the 3L+ FL cohort, and an ORR of 92% with all responses being CRs in the 2L cohort. Among patients in the liso-cel treated set who received bridging therapy, the ORR was 95% (38/40) with all responses being CRs in the 3L+ FL cohort, and 80% (4/5) with all responses being CRs in the 2L cohort.
Safety
Among patients treated with liso-cel, 97 (75%) experienced ≥Grade 3 treatment-emergent adverse events (TEAEs), and 32 (25%) experienced serious TEAEs. The most common ≥Grade 3 TEAEs were cytopenias, including neutropenia in 76 patients (58%), and anemia and thrombocytopenia in 13 patients (10%) each (Table 2). Eight patients (6%) experienced febrile neutropenia.
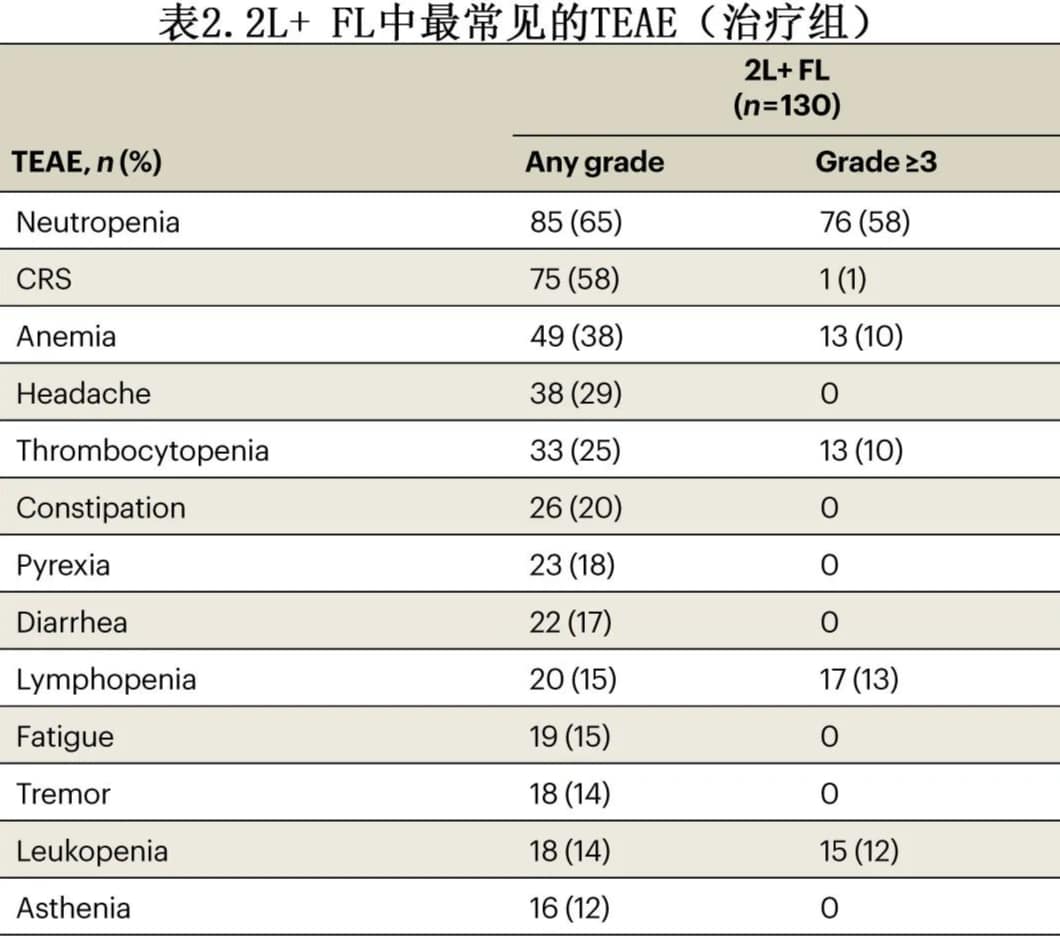
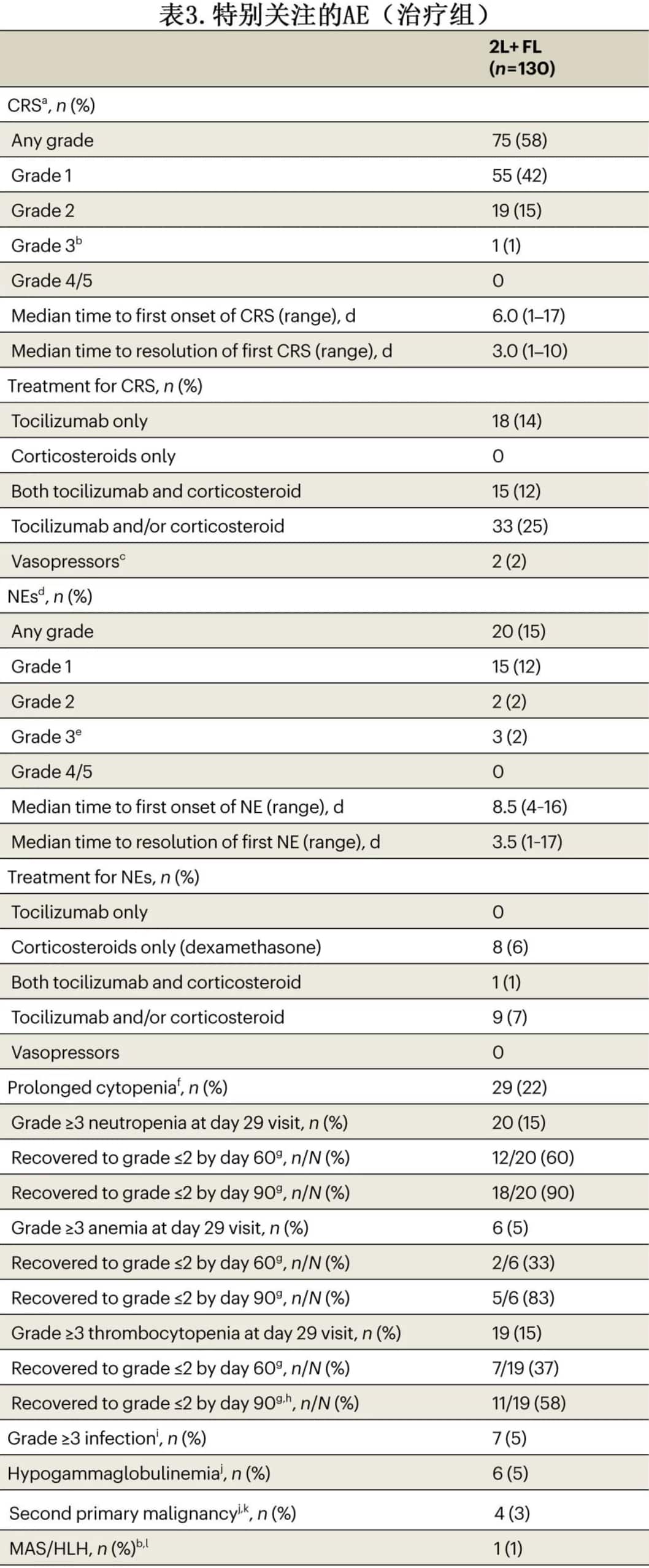
Seventy-five patients (58%) experienced cytokine release syndrome (CRS), with a median time to onset of 6 days and a median duration of 3 days; most cases were Grade 1, with only 1 Grade 3 case and no Grade 4-5 cases. Twenty patients (15%) experienced neurological events, with a median time to onset of 8.5 days and a median duration of 3.5 days; most cases were Grade 1, with 3 Grade 3 cases and no Grade 4-5 cases. Seven patients (5%) experienced ≥Grade 3 infections within 90 days post-treatment, with an additional 3 patients experiencing late infections. Twenty-nine patients (20%) experienced prolonged cytopenia (≥Grade 3 cytopenia occurring >29 days). Six patients (5%) experienced hypogammaglobulinemia. There were 13 deaths during the study, including 4 due to disease progression. Macrophage activation syndrome/hemophagocytic lymphohistiocytosis (MAS/HLH) and second primary malignancies occurred in 1 patient (1%) and 4 patients (3%), respectively.
Summary
The TRANSCEND phase 2 study was the first to evaluate CAR-T therapy in second-line (2L) relapsed/refractory (R/R) follicular lymphoma (FL), enrolling patients with 2L+ R/R FL, with 2L patients required to meet criteria for POD24 (progression of disease within 24 months after first-line therapy) and/or ≥1 modified GELF (mGELF) criteria. A total of 130 patients were enrolled (median follow-up of 18.9 months). In the 3L+ FL cohort (n=101), the overall response rate (ORR) was 97% with a 94% complete response (CR) rate; in the 2L FL cohort (n=23), the ORR was 96%, all achieving CR. Fifty-eight percent of patients experienced cytokine release syndrome (≥Grade 3 in 1%); 15% of patients experienced neurological events (≥Grade 3 in 2%).
Overall, liso-cel demonstrated efficacy and safety in patients with R/R FL, including those with high-risk 2L disease.
References
Morschhauser F,et al. Lisocabtagene maraleucel in follicular lymphoma: the phase 2 TRANSCEND FL study.Nat Med . 2024 Jun 3. doi: 10.1038/s41591-024-02986-9.
Content Source:聊聊血液
