The First Human Trial of Autologous CD5.CAR-T for T Cell Lymphoma Without Gene Editing
The First Human Trial of Autologous CD5.CAR-T for T Cell Lymphoma Without Gene Editing
T cell lymphomas (TCLs) are more challenging hematologic malignancies compared to B cell lymphomas. Despite recent therapeutic advances, the prognosis for refractory or relapsed TCL patients remains poor, with a 3-year event-free survival rate below 30% across subtypes. In this setting, hematopoietic stem cell transplantation (HSCT) offers the only prospect of long-term disease-free survival, but the outcome is dismal for those relapsing after allogeneic HSCT.
Chimeric antigen receptor (CAR)-modified T cells have successfully treated B cell lymphomas, but applying a similar approach to TCLs is more challenging due to the complexity of targeting T cell antigens. Expression of T cell antigens on CAR-T cells may lead to fratricide, impairing ex vivo expansion; moreover, persistent in vivo activity of CAR-T cells could increase patient risks such as T cell deficiency and infectious complications.
CD5 is widely expressed in T cell malignancies, including T cell lymphomas. In December 2023, the journal Blood published an article titled “Anti-tumor Efficacy and Safety of Unedited Autologous CD5.CAR T cells in Relapsed/Refractory Mature T-cell Lymphomas” by LaQuisa Hill’s research team from Baylor College of Medicine. They developed a CD5.CAR-T cell product that downregulated CD5 protein levels on transduced T cells, reducing fratricide while maintaining cytotoxicity against CD5-positive tumor cells. The researchers conducted a first-in-human phase 1 trial to evaluate the safety and efficacy of unedited autologous CD5.CAR-T cells in treating relapsed/refractory (r/r) CD5+ mature T cell malignancies, and this paper reports the clinical outcomes in the mature TCL patient cohort.

Results
Baseline Patient Characteristics
The study enrolled 17 T cell lymphoma patients, successfully manufacturing CD5.CAR-T cells for 13/14 patients (93%) and infusing 9 patients (69%). At three dose levels (DL1: 1×10^7; DL2: 5×10^7; DL3: 1×10^8 CAR+ cells/m2), patients received either a single dose (n=7) or two doses (n=2) of CD5.CAR-T cells. There were 5 male and 4 female patients, with a median age of 63 years (range: 29-71 years), having received a median of 5 prior lines of therapy (range: 2-18). Three patients had prior autologous HSCT, and 2 relapsed post allo-HSCT. The median 75% (range, 51-100%) of tumor cells expressed CD5 by multicolor flow cytometry (n=6) or IHC (n=3). The median time from enrollment to infusion was 56 days (range: 39-81 days).
Table 1. Baseline characteristics of treated patients
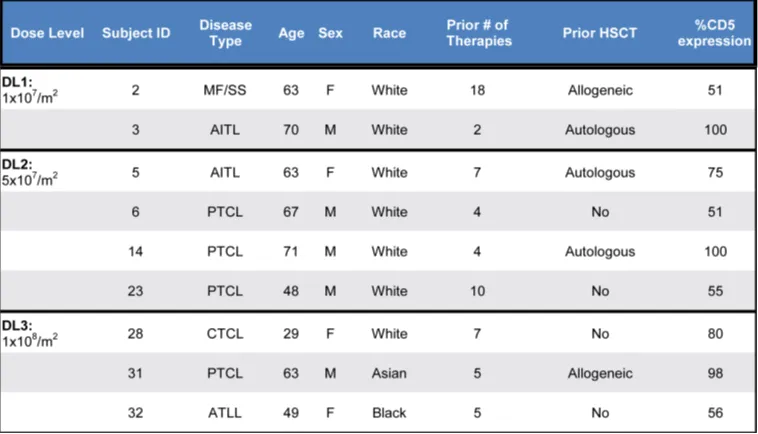
CD5.CAR-T Cell Products
Table 2 summarizes the characteristics of the CAR-T cell products. CD5.CAR-T cells were manufactured using either the “standard” expansion protocol (6-7 days from transduction to cryopreservation) or a shortened protocol (4-5 days), with a median manufacturing time of 5 days (total 8 days) from lentiviral transduction to cryopreservation, plus 14 days for sterility testing. The median fold expansion of CD5.CAR-T cells from transduction to cryopreservation was 23.4 (range 9.2-133.3), with an average daily expansion of 4.1-fold (range 2.3-19). At cryopreservation, >97% of T cells expressed the CD5.CAR, with CD4/CD8 ratios ranging from 0.1 to 1.7. No transduced malignant T cells were detected by flow cytometry. The median vector copy number per transduced T cell was 4.6 (range 1.62-7.71).
Table 2. Characteristics of infused T cell products
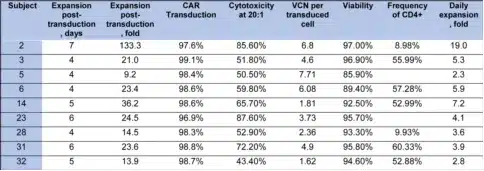
Safety
CD5.CAR-T cell therapy was well-tolerated in lymphoma patients. All treated patients did not experience any infusion-related toxicities within 4 hours of infusion. The major adverse events were cytopenias driven by lymphodepletion (Table 3), with most patients (6/9) recovering normal counts by day 28. Other common adverse events included fatigue, headache, elevated transaminases, and non-neutropenic fever. A few patients experienced severe adverse events such as hyperuricemia and pericardial effusion, but these may have been related to disease progression.
The incidence of CAR-T cell-related cytokine release syndrome (CRS) was relatively low, with all cases being grade ≤2 and short-lived. One patient developed grade 2 neurotoxicity on day 4 post-infusion, accompanied by grade 2 CRS, which resolved with supportive care. Infectious complications were limited to catheter-related bacteremia responsive to antibiotics (n=2), with one patient having a concomitant urinary tract infection, and another patient experiencing BK virus and cytomegalovirus (CMV) reactivation.
CD5.CAR-T cells did not completely eliminate normal T cells from the peripheral blood and lymph nodes, with circulating CD4+ and CD8+ T cells persisting as CAR-T cell levels waned, comprising 7.7%-89.9% of total lymphocytes (Figure 1A-C).
Table 3. Treatment-related adverse events at least possibly related to study drug
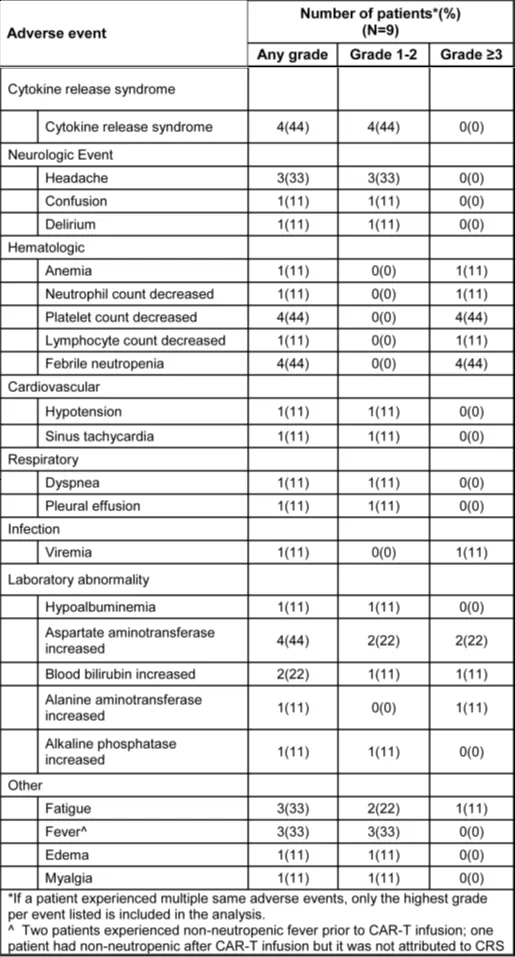
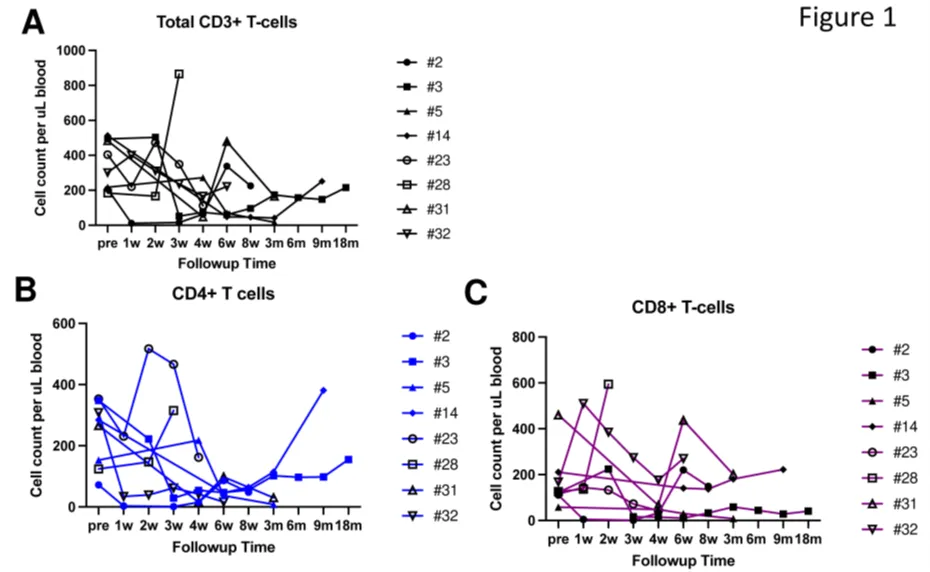
Figure 1. Circulating T cells following CD5.CAR-T cell infusion. Levels of total CD3+ T cells (A), CD3+CD4+ cells (B), and CD3+CD8+ cells (C) in the peripheral blood of patients following CD5.CAR-T cell infusion.
Clinical Responses
Objective clinical responses were observed in 4/9 patients (44%) across all dose levels. Four patients experienced progressive disease (PD) following infusion, patients #2 (mycosis fungoides/Sezary syndrome), #6, #23, and #28 (PTCL), all of whom died from PD or PD-related complications 4-17 weeks after CAR-T cell infusion. One PTCL patient (#32) had stable disease (SD) on the initial disease assessment post CAR-T cell infusion but died 9 months later after receiving additional salvage therapy, although in CR at the time of death. One patient with HTLV-1-driven adult T cell lymphoma with massive cervical and mediastinal involvement (#31; treated at DL3) achieved a partial response (PR), with PET-CT showing a marked reduction in the left supraclavicular lymph node mass from 11.8 cm x 8.8 cm to 3.3 cm x 2.4 cm with no abnormal uptake.
Overall, 3 patients died from lymphoma progression within 60 days of CAR-T cell infusion (1 within 30 days). All deaths were not attributed to the investigational product or study procedures. Of the remaining 6 patients, 4 died at 4-15 months, while 2 remained alive at 41 and 48 months (Figure 2). In patients who underwent biopsy at the time of disease progression or relapse (n=7), no loss of CD5 expression on tumor cells was observed.
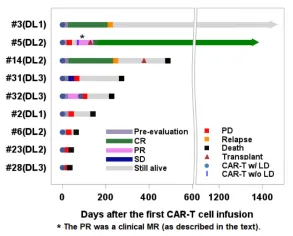
Figure 2. Swimmer plot for the mature T cell lymphoma cohort.
The expansion and persistence of CD5.CAR-T cells
The PK results showed that CD5.CAR-T cells could be detected in the peripheral blood of all patients starting 3 hours after infusion. The CAR transgene copy numbers reached a peak from week 1 to week 3, with an average peak of 9300 transgene copies/mL, and then gradually decreased, with the longest detection up to 9 months. Two patients who received a second infusion of CD5.CAR-T cells still had detectable transgenic cells at 8 weeks after infusion, one of whom (subject 5) did not receive lymphodepletion prior to re-infusion. There was no significant difference in the peak levels and persistence of CD5.CAR-T cells among different dose levels, responders, and non-responders.
When treating patients with CD5.CAR-T cell products with a shortened manufacturing time, objective clinical responses were observed in all patients. Compared with products using the standard manufacturing process, the products with a shortened manufacturing time showed higher expansion and persistence. The shortened manufacturing time was associated with an increased frequency of minimally differentiated CD4+ and CD8+ T cells co-expressing CD62L and CCR7, as well as an increase in CD27+ T cells.
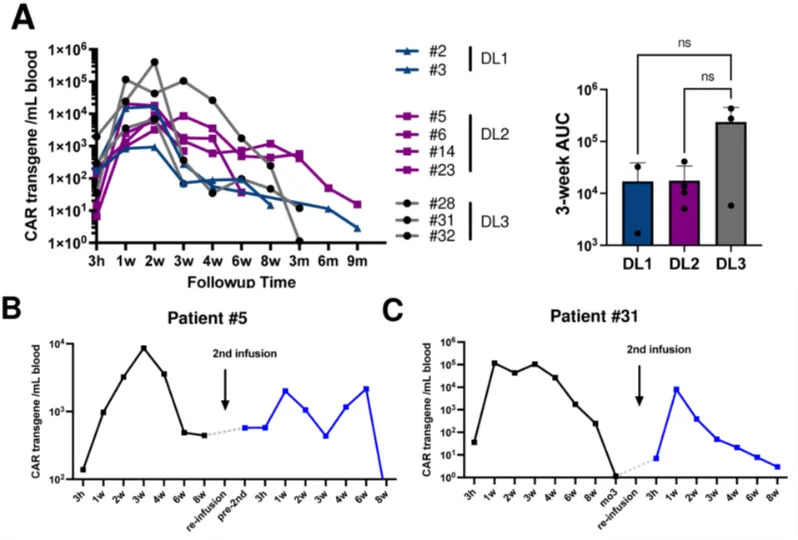
Figure 3. Expansion and persistence of CD5 CAR-T cells after infusion. (A) CD5 CAR transgene levels per milliliter of blood. The area under the curve (AUC) for the first 3 weeks at each dose level is plotted in the bar graph. CD5 CAR transgene levels for subjects #5 (B) and #31 (C) who received a second dose of CD5 CAR-T cells.
Conclusion
This report describes the first-in-human trial of the safety and efficacy of unedited autologous CD5.CAR-T cell therapy for relapsed/refractory mature r/r T-cell lymphomas, including various types of refractory T-cell lymphomas (including PTCL, MF/SS, AITL, and HTLV1-related ATLL). The study showed that treatment with autologous unedited second-generation CD5.CAR-T cells in these patients was safe, and no selective T-cell deficiency (CD4+ absolute cell count <200 for 8 weeks) or resistance mechanisms such as target antigen loss were observed.
At all dose levels, CD5.CAR-T cells underwent expansion, and in patients achieving CR, the persistence lasted more than 6 months. Furthermore, the study found that CD5.CAR-T cells could induce objective clinical remissions, including CRs, PRs, and MRs. Finally, the study demonstrated the role of naive-like and central memory T cells in enhancing systemic CD5.CAR-mediated anti-lymphoma activity, but further research is needed for verification. Despite some challenges in subsequent treatment, this phase I clinical trial showed that CD5.CAR-T cell therapy as a treatment option for r/r mature TCL is safe and feasible, and may be considered for use in earlier stages of the disease.
References
[1] LaQuisa C Hill, Rayne H Rouce, Mengfen Wu, Tao Wang, Royce Ma, Huimin Zhang, Birju Mehta, Natalia Lapteva, Zhuyong Mei, Tyler S Smith, Lina Yang, Madhuwanti Srinivasan, Phillip M Burkhardt, Carlos A Ramos, Premal D Lulla, Martha Arredondo, Bambi Grilley, Helen E Heslop, Malcolm K Brenner, Maksim Mamonkin. Anti-tumor Efficacy and Safety of Unedited Autologous CD5.CAR T cells in Relapsed/Refractory Mature T-cell Lymphomas. 2023 Dec 25:blood.2023022204. doi: 10.1182/blood.2023022204.
Content Source:细胞知聊
