CAR-T Immunotherapy for Lymphoma: 80-year-old Patient Achieves Cure After 10 Years of Treatment
CAR-T Immunotherapy for Lymphoma: 80-year-old Patient Achieves Cure After 10 Years of Treatment
In 2021, the approval of CAR-T cell therapy in China caused a huge sensation and attention, bringing hope for survival to patients with diffuse large B-cell lymphoma and becoming one of the most anticipated therapies for cancer patients. Recently, the Department of Biological Therapy at the Tumor Medicine Department of the Chinese PLA General Hospital welcomed the return visit of one of the first CAR-T cell therapy patients who received treatment 10 years ago and achieved a cure.

In the early spring warmth of March 2024, upon entering the ward of the Department of Biological Therapy on the 12th floor of the Oncology Building at the First Medical Center of the PLA General Hospital, an 80-year-old patient with a vigorous spirit was exchanging treatment experiences with fellow patients during a break from examination. In the ward, the energetic elderly gentleman was encouraging a slightly weaker “younger person” nearby with his own experience, creating a special scene. He happily told those around him, “This year marks the 10th anniversary since CAR-T cells cured me!”
This elderly “healthy” patient, Mr. Zhang, was once a patient with refractory diffuse large B-cell lymphoma. Despite undergoing dozens of chemotherapy cycles and local radiotherapy, his condition continued to progress.
In 2013, at the age of 70, he sought new treatment options and visited the Department of Biological Therapy at the First Medical Center of the PLA General Hospital. After undergoing comprehensive examinations and repeated evaluations by the clinical team led by Professor Han Weidong and Associate Chief Physician Liu Yang, the decision was made to administer CAR-T cell therapy to Mr. Zhang—enrolling him in the CAR-T cell clinical research study, making him one of the first CAR-T cell therapy patients in China.
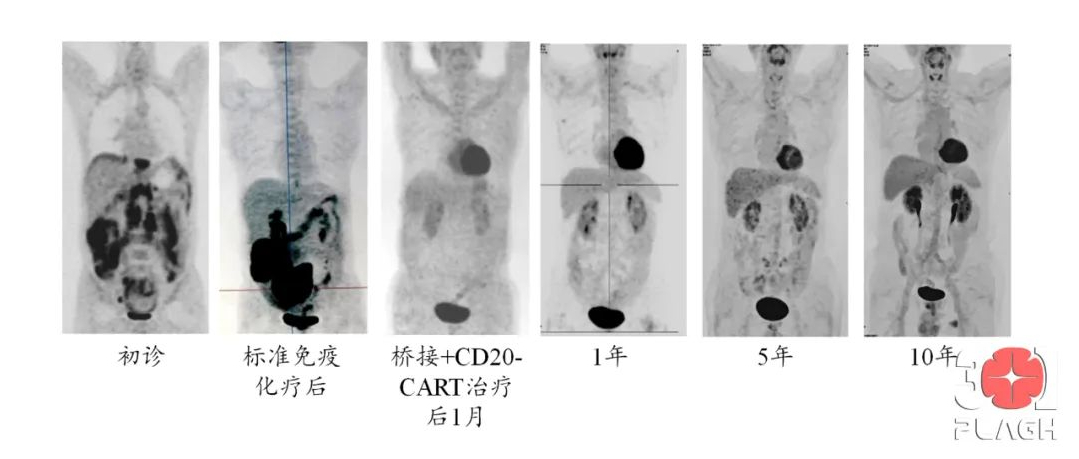
In February 2013, Mr. Zhang successfully underwent cell reinfusion. One month after reinfusion, he achieved complete remission, and regular follow-ups showed that he remained in complete remission 10 years later, truly achieving a cure for his cancer. Mr. Zhang returns annually for regular visits to see the medical staff who once treated him and has become a special “healthy” long-term patient at the Department of Biological Therapy.
In 2012, the Department of Biological Therapy initiated China’s first CAR-T clinical trial using its independently developed technology. Mr. Zhang was one of the first patients (the third case), and the first paper was published in Clinical Immunology in 2014, with further follow-up results published in STTT in 2016 and 2017.
This team was the earliest in China to use independently developed CAR-T technology to treat lymphoma patients. This patient has achieved the longest disease-free survival after CAR-T treatment in China, further confirming the “curative potential” of CAR-T technology for lymphoma patients. During this period, they completed the use of independently developed CAR-T technology, obtained China’s first CAR-T patent, and became the first in the world to use CAR-T technology targeting CD20 to treat patients with diffuse large B-cell lymphoma (DLBCL), an invasive type of lymphoma.
Building on the clinical research targeting a single tumor antigen, the Department of Biological Therapy continued to deepen its efforts in this field, continuously developing new generations of anti-tumor immunocellular therapies and conducting clinical studies to further enhance treatment efficacy.
In recent years, with support from multiple national-level research projects, the team has independently developed dual-targeted and epigenetically modified CAR-T cells for the treatment of advanced lymphoma patients. The complete remission rate exceeded 70%, and the median disease-free survival period exceeded 27 months, significantly outperforming the previous single-target clinical efficacy, bringing hope for “rebirth” to more lymphoma patients. The research results have been successively published in internationally renowned academic journals such as Blood, Nature Communications, JHO, and Leukemia.
Content source:全意生物工程研究院
