Recent Advances in CAR-T Lymphoma Treatment
Recent Advances in CAR-T Lymphoma Treatment
In recent years, CAR-T cell therapy has been continuously developing and has made significant progress in the field of lymphoma. The 29th Annual Congress of the European Hematology Association (EHA) was held in Madrid, Spain, from June 13 to 16, 2024. At the conference, the latest advances in CAR-T cell therapy for relapsed/refractory (R/R) lymphoma were reported. Here, I have compiled a summary for our readers.
Axicabtagene Ciloleucel (axi-cel) Can Provide Durable Remission for Patients with R/R Primary Mediastinal B-Cell Lymphoma (PMBCL)
PMBCL is a rare subtype of large B-cell lymphoma (LBCL) with a favorable prognosis after first-line immunochemotherapy, but R/R patients have a poor prognosis. CAR-T cell therapy has demonstrated impressive complete response (CR) rates and progression-free survival (PFS) in R/R LBCL. This study aimed to evaluate the efficacy and safety of CD19 CAR-T therapy in patients with R/R PMBCL.
From October 2018 to March 2023, 69 patients with PMBCL who received CD19 CAR-T therapy were included. The median age of the patients was 34 years (with 3 patients over 60 years old). The median number of prior lines of therapy was 2, with 6 patients receiving only one line of treatment and 7 patients receiving ≥4 lines of treatment. The study analyzed patients’ baseline characteristics, bridging therapy, toxicity, response rates, PFS, and overall survival (OS).
Patients received either axi-cel (n=55) or tisagenlecleucel (tisa-cel) (n=14) treatment. Fifty-eight (84%) patients received bridging therapy, with 12 (17%) receiving radiation therapy, 20 (29%) receiving immune checkpoint inhibitor therapy, and 30 (52%) achieving remission before CAR-T infusion.
The median follow-up time of the study was 20 months. Among patients receiving axi-cel, the CR and partial response (PR) rates were 80% and 11%, respectively, with a 1-year PFS rate of 69% (95% CI: 58.6%-83.1%) and a 1-year OS rate of 86.5% (95% CI: 79.7%-99.2%). Among the evaluable patients receiving tisa-cel (n=12), only 4 patients (33%) achieved CR, and 4 (33%) achieved PR, with a 1-year PFS rate of 14% and an OS rate of 49%.
This study represents the largest cohort of patients with R/R PMBCL treated with CD19 CAR-T therapy to date. The results showed that axi-cel has high efficacy in R/R PMBCL, with favorable outcomes even in patients who did not respond well to bridging therapy.
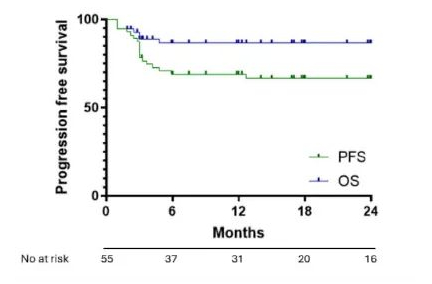
Figure 1: OS and PFS with axi-cel treatment in R/R PMBCL
CD19 CAR-T Therapy May Be a Treatment Option for Elderly Patients with R/R LBCL
CD19 CAR-T has transformed the treatment landscape for R/R large B-cell lymphoma (LBCL). However, elderly patients face unique challenges, including immune senescence limiting efficacy and a higher burden of comorbidities increasing toxicity. As patients aged ≥75 years were underrepresented in clinical trials, the LYSA study aimed to explore the efficacy of CD19 CAR-T therapy in patients with R/R LBCL (≥75 years) who had received ≥3 prior lines of therapy.
This retrospective study analyzed 1524 patients with R/R LBCL who had received ≥2 lines of therapy between April 2018 and September 2023, with a focus on patients aged ≥75 years. The primary endpoint was OS, and secondary endpoints included PFS, best objective response rate (ORR) and CR rate, incidence of ≥grade 3 cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), and non-relapse mortality (NRM, defined as death from causes unrelated to lymphoma relapse/progression; deaths from unknown causes were excluded).
Patients received either axi-cel (n=1065, 69.8%) or tisa-cel (n=459, 30.1%) treatment. There were 125 patients aged ≥75 years (median age 76 years, range 75-78 years) and 1399 patients aged <75 years (median age 62 years, range 58-75 years). The two groups were well-balanced in terms of sex, LBCL subtype, number of prior lines of therapy, performance status, age-adjusted International Prognostic Index, proportion of patients receiving bridging therapy, and response to bridging therapy. However, patients aged ≥75 years had higher HCT-CI scores and were less likely to have previously undergone transplantation compared to those aged <75 years.
With a median follow-up of 12.7 months (95% CI, 9.6 months-21.2 months), 457 patients were evaluable for efficacy. The best ORR/CR rates were 74.8%/62.6% in the ≥75 years group and 78.0%/60.8% in the <75 years group (p=0.425/0.699). The estimated median OS was 18.3 months in the ≥75 years group and 24.0 months in the <75 years group (p=0.12), while the estimated median PFS was 8.2 months and 6.1 months, respectively (p=0.73). The incidence of ≥grade 3 CRS and ICANS was 7.3%/7.4% (p=0.97) and 9.8%/12.4% (p=0.39) in the ≥75 years and <75 years groups, respectively, with no statistically significant difference.
In the safety analysis, the ≥75 years group had a higher NRM rate, with 20.0% of deaths unrelated to lymphoma progression/relapse compared to 8.7% in the <75 years group (p<0.001). Three patients aged ≥75 years and 16 patients aged <75 years experienced early NRM (within 28 days of infusion).
This real-world study demonstrated that CD19 CAR-T therapy in patients aged ≥75 years did not significantly differ in efficacy and survival compared to younger patients. However, the higher NRM rate in the elderly population warrants further investigation and may inform treatment selection for these patients.
Content source:医学界血液频道
