Nat commun: Zhaoweili’s Team from Shanghai Jiao Tong University Found the Mechanism of Drug Resistance in the Treatment of Diffuse Large B Cell Lymphoma CAR-T
Nat commun: Zhaoweili’s Team from Shanghai Jiao Tong University Found the Mechanism of Drug Resistance in the Treatment of Diffuse Large B Cell Lymphoma CAR-T
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous B-cell lymphoma, typically presenting as an aggressive or advanced-stage disease, accounting for 30-40% of all newly diagnosed non-Hodgkin’s lymphomas. Chemotherapy combined with Rituximab (CD20-targeted antibody) can significantly improve the prognosis of DLBCL patients. However, approximately 20% of patients with primary refractory DLBCL have a poor prognosis, with a median overall survival (OS) of only 7.1 months.
Chimeric antigen receptor T (CAR-T) cell therapy has shown promising efficacy in early trials for relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Several studies have demonstrated the efficacy and manageable adverse reactions of CD19-targeted chimeric antigen receptor T (CAR-T) cell therapy for the treatment of relapsed/refractory DLBCL. However, its efficacy in treating primary refractory DLBCL has not been comprehensively studied, and the potential mechanisms of resistance are still unclear.
On June 18, 2024, the team led by Zhao Weimu from Shanghai Jiao Tong University published a research paper titled “Cholesterol efflux from C1QB-expressing macrophages is associated with resistance to chimeric antigen receptor T cell therapy in primary refractory diffuse large B cell lymphoma” in Nature Communications online. The paper reported the results of a phase I, open-label, single-arm clinical trial, with relmacabtagene autoeucel (relma-cel), a CD19-targeted CAR-T cell product, as the primary endpoint for safety and efficacy.

Among the 12 enrolled patients, 8 patients experienced grade 4 hematological toxicity-related adverse events. No ≥ grade 3 cytokine release syndrome or neurotoxicity occurred. Single-cell RNA sequencing revealed that the proportion of C1QB-expressing macrophages increased in patients with progressive disease before CAR-T cell therapy. The study found that cholesterol efflux from M2 macrophages induced an immunosuppressive state in CD8+ T cells, leading to their exhaustion and thereby inhibiting the cytotoxicity of CAR-T cells. Potential interactions between macrophages and CD8+ T cells, mediating lipid metabolism (AFR1-FAS), immune checkpoint activation, and T cell exhaustion (LGALS9-HAVCR2, CD86-CTLA4, and NECTIN2-TIGIT) were enhanced in disease progression. These findings suggest that cholesterol efflux from macrophages may trigger CD8+ T cell exhaustion, providing a rationale for metabolic reprogramming to counteract CAR-T therapy failure.
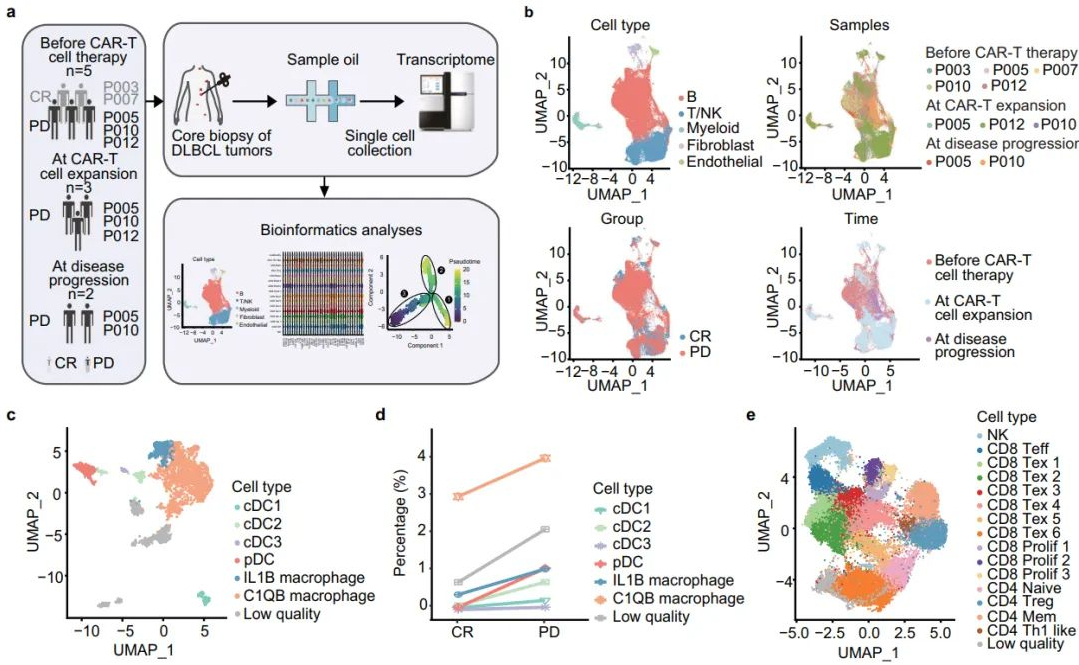
Tumor microenvironment characteristics of CR and PD patients before CAR-T cell therapy
Image source | Nature Communications
Conclusion
The tumor microenvironment (TME) plays a crucial role in the resistance process to CAR-T cell therapy. The immunosuppressive nature of the TME promotes CAR-T cell exhaustion, which is a major obstacle to clinical treatment efficacy. It has been reported that metabolic reprogramming of M2 macrophages can modulate the efficacy of immunotherapy and T cell function. In fact, cholesterol has been shown to induce immune checkpoint expression and CD8+ T cell exhaustion within the TME. However, the specific metabolic features of the TME, particularly those of M2 macrophages, in DLBCL patients after CAR-T cell therapy remain highly intriguing.
Relmacabtagene autoeucel (relma-cel) is a CD19-targeted CAR-T cell product with a 4-1BB costimulatory domain structure. A phase I study confirmed the preliminary safety and efficacy of CAR-T cell therapy for r/r DLBCL. This finding supported the rational design and initiation of a phase I, single-arm, open-label, multicenter clinical trial (JWCAR029-003) of relma-cel treatment for primary refractory DLBCL.
The study results demonstrated the safety and efficacy of CAR-T cell therapy in patients with primary refractory DLBCL. Additionally, the study utilized single-cell RNA sequencing (scRNA-seq) to explore the mechanisms of resistance to CAR-T cell therapy in these patients.
Reference Material
https://www.nature.com/articles/s41467-024-49495-4
Content source:上海细胞治疗工程技术研究中心
