DLBCL Kymriah was Approved for Listing on May 1, 2018
DLBCL Kymriah was Approved for Listing on May 1, 2018
On May 1, 2018, the U.S. Food and Drug Administration (FDA) approved Novartis’ Tisagenlecleucel (Kymriah) for the treatment of adult patients with relapsed or refractory large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma (FL).

1. Background of Kymriah’s Approval
Kymriah is a CD19-targeted chimeric antigen receptor T-cell (CAR-T) immunotherapy. In August 2017, Kymriah became the first cell-based gene therapy approved by the FDA, for the treatment of patients up to 25 years old with refractory or relapsed (>2 times) B-cell precursor acute lymphoblastic leukemia (ALL).

Clinical Trial
The open-label, multicenter, single-arm Phase II “JULIET” clinical study enrolled 160 patients with relapsed or refractory DLBCL from 27 study centers in the United States, Canada, Australia, Japan, and Europe. These patients had received at least two prior lines of chemotherapy, including rituximab and an anthracycline, or had relapsed after autologous hematopoietic stem cell transplantation (HSCT).
The results showed that the overall response rate (ORR) in adult patients with relapsed or refractory DLBCL treated with Kymriah was 50% (95% CI, 38%-62%), with a complete response rate (CR) of 32% and a partial response rate of 18%.
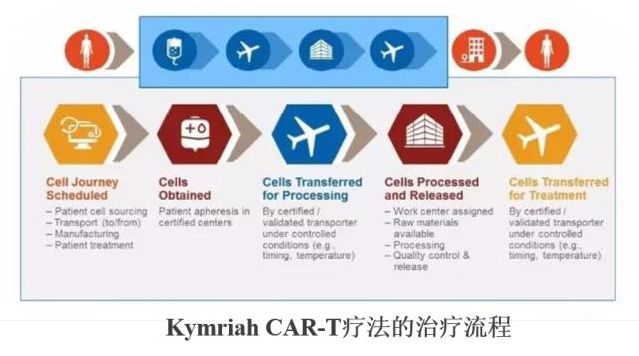
2. Features of Kymriah
Kymriah is not indicated for the treatment of primary central nervous system lymphoma. As the first FDA-approved chimeric antigen receptor T-cell (CAR-T) therapy, it is also the only CAR-T cell therapy approved by the FDA for two different indications: non-Hodgkin’s lymphoma (NHL) and B-cell ALL.
Kymriah, a CAR-T immunotherapy, was co-developed by the University of Pennsylvania and Novartis. Novartis’ decision to collaborate with the University of Pennsylvania was well-founded. Since 2000, the U.S. federal government has consistently funded CAR-T research, with the laboratory of Carl June at the University of Pennsylvania receiving the most significant funding support.

3. Best Institutions or Hospitals for Lymphoma Treatment
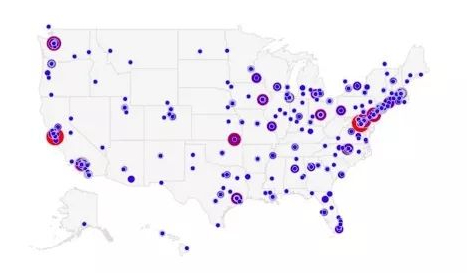
An analysis of lymphoma research data reveals that since 2000, the U.S. federal government has invested approximately $5.7 billion in this field, supporting 14,792 projects at 639 institutions. The research involves approximately 3,435 experts, whose geographical distribution is shown in the following map:
Assuming you are a professional medical translator, proficient in both medical knowledge and English-Chinese translation, capable of accurately translating medical terminology, you are now tasked with precisely translating the above article into English. Ensure that medical terms are accurately translated, the meaning is clear, and all content, including references to images (e.g., “Image X”), is preserved without unauthorized omissions.
References
FDA official website
Official website of Novartis pharmaceuticals Corp
Content Source:乐美好医
