Approved Indications for EMA Yescarta Diffuse Large B-cell Lymphoma (DLBCL) and Advanced B-cell Lymphoma (HGBL) on October 17, 2022
Approved Indications for EMA Yescarta Diffuse Large B-cell Lymphoma (DLBCL) and Advanced B-cell Lymphoma (HGBL) on October 17, 2022
On October 17, 2022, exciting news came from the European Commission (EC). Based on the data from the ZUMA-7 study, the EC officially approved a new indication for Yescarta® for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or high-grade B-cell lymphoma (HGBL) after one line of chemoimmunotherapy failed or within 12 months of first-line chemoimmunotherapy[1]. This is the first CAR-T cell therapy approved in the EU for the second-line treatment of large B-cell lymphoma (LBCL), bringing a new treatment option for lymphoma patients in Europe!
DLBCL is the most common type of non-Hodgkin lymphoma (NHL), accounting for approximately 25% to 50% of all NHLs[2]. Even after first-line treatment, 30% to 40% of DLBCL patients may still experience relapse or become refractory[3], and once progressed to relapsed or refractory disease, the median survival may be only 6.3 months[4]. HGBL is a heterogeneous disease with morphological and genetic features between DLBCL and Burkitt lymphoma[5], accounting for 1% to 2% of all NHLs[6]. HGBL is characterized by high aggressiveness, rapid disease progression, poor prognosis, and poor response to conventional chemotherapy regimens, with a relatively low cure rate[6]. Both DLBCL and HGBL patients have limited benefit from second-line treatments, and there is an urgent need for breakthrough therapies.
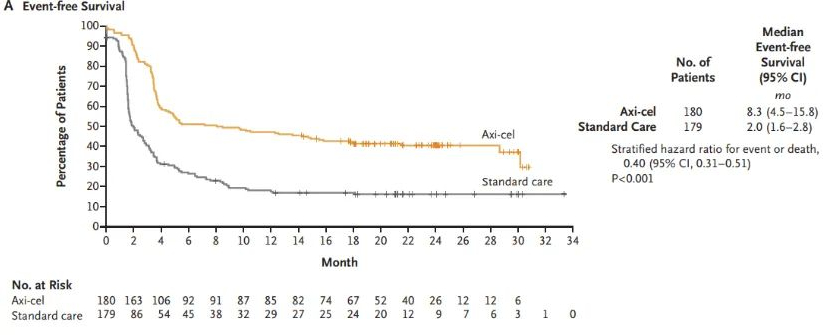
Yescarta® (generic name in the US: axicabtagene ciloleucel, abbreviated as Axi-Cel) is a gene-modified autologous T-cell immunotherapy targeting CD19[7]. In August 2018, Yescarta® was first approved in the EU for the treatment of adult patients with relapsed or refractory (R/R) LBCL after two or more lines of systemic therapy, including DLBCL and primary mediastinal large B-cell lymphoma (PMBCL)[8]. In the same year, Yescarta® initiated the ZUMA-7 study to compare the efficacy and safety of Axi-Cel or standard therapy for second-line treatment of LBCL. ZUMA-7 is a global, multicenter, randomized phase III study that enrolled 359 patients with LBCL who either failed to respond to first-line chemoimmunotherapy or relapsed within 12 months after first-line chemoimmunotherapy. Patients were randomly assigned 1:1 to receive Axi-Cel (n=180) or standard therapy (n=179), with the primary endpoint being event-free survival (EFS)[9]. At a median follow-up of 24.9 months, the median EFS was significantly longer in the Axi-Cel group compared to the standard therapy group (8.3 months vs. 2.0 months, HR: 0.40 [95% CI, 0.31–0.51], P<0.001) (Figure 1). The 24-month EFS rate was also higher in the Axi-Cel group compared to the standard therapy group (41% vs. 16%)[9].
Figure 1: EFS data for Axi-Cel vs. Standard Therapy
For DLBCL patients, Axi-Cel reduced the risk of disease progression or death by 56% compared to standard therapy (HR: 0.44 [95% CI, 0.32–0.60]); for HGBL patients (including those with MYC rearrangement with BCL-2 and/or BCL-6 rearrangement), Axi-Cel reduced the risk of disease progression or death by 72% compared to standard therapy (HR: 0.28 [95% CI, 0.14–0.59]) (Figure 2)[9].
Figure 2: Forest plot for key subgroups (Axi-Cel vs. Standard Therapy)

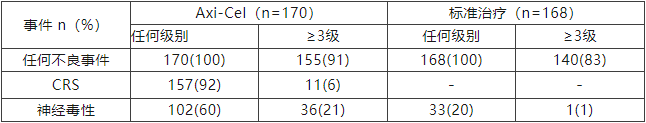
The safety profile of Axi-Cel in the ZUMA-7 study was consistent with previous related studies[1]. The incidence of grade ≥3 adverse events was 91% in the Axi-Cel group and 83% in the standard therapy group. Among patients receiving Axi-Cel, the incidence of grade ≥3 cytokine release syndrome (CRS) and neurological toxicity events was 6% and 21%, respectively (Table 1), with no CRS or neurological toxicity-related deaths[9].
Table 1: Incidence of Adverse Events (Axi-Cel vs. Standard Therapy)
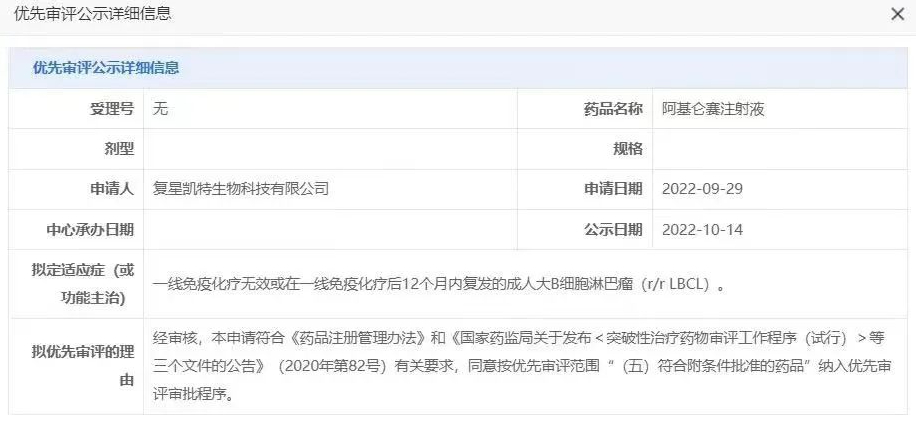
Based on the significant survival benefit demonstrated in the ZUMA-7 study, in April 2022, the FDA first approved Yescarta® for the new second-line LBCL treatment indication (for the treatment of adult patients with LBCL that is refractory to or relapses within 12 months after first-line chemoimmunotherapy)[10], followed by the recent EU approval for the same new indication. 奕凯达 (Axi-Cel injection, 奕凯达®、阿基仑赛注射液) is the locally produced autologous anti-CD19 CAR-T cell therapy product licensed from Kite Pharma by Fosun Kite, and was officially approved by the NMPA in China on June 23, 2021. On October 14, 2022, the CDE announced that the marketing application for the second-line LBCL treatment indication of Yikada is proposed to be included in the priority review[11] (Figure 3). We look forward to the early approval of the new second-line LBCL treatment indication for Yikada, bringing new hope for a cure to LBCL patients and families in China!
Figure 3: CDE announcement on the status of the marketing application for the second-line LBCL treatment indication of 奕凯达
Reference Material
[1] Kite’s Yescarta First CAR T-cell Therapy to Receive European Marketing Authorization for Use in Second-Line Diffuse Large B-cell Lymphoma and High-grade B-cell Lymphoma (kitepharma.com)
[2] 弥漫性大B细胞淋巴瘤诊疗指南(2022年版).
[3] Zahid U, et al. Curr Hematol Malig Rep. 2017 Jun;12(3):217-226.
[4] Crump M, et al. Blood. 2017;130(16):1800-1808.
[5] 贺妍, 等. 白血病 · 淋巴瘤. 2017; 26(3):142-144.
[6] 白银银, 等. 白血病 · 淋巴瘤. 2020; 29(12):765-768.
[7] 阿基仑赛注射液说明书
[8] Yescarta® (axicabtagene ciloleucel) EMA说明书
[9] Locke FL, et al. N Engl J Med. 2022;386(7):640-654.
[10] Yescarta® (axicabtagene ciloleucel) FDA说明书
[11] https://www.cde.org.cn/main/xxgk/listpage/2f78f372d351c6851af7431c7710a731
Content Source:复星医药
