Why FDA Tecartus Suddenly Deleted the Warning Letter on January 23, 2024
Why FDA Tecartus Suddenly Deleted the Warning Letter on January 23, 2024
After the FDA issued a safety labeling change notification to all commercial CAR-T therapy manufacturers, Tecartus received some leeway from the regulatory agency’s warning list for BCMA and CD19-directed gene-modified autologous T-cell immunotherapies. These therapies were supposed to carry a boxed warning about the risk of secondary malignancies.
On Friday, the FDA sent letters to the manufacturers of six approved CAR-T products (including Gilead), requiring them to add a class-wide boxed warning to their respective labels, alerting patients and prescribing physicians to the potential risk of T-cell malignancies, including serious consequences such as hospitalization and death.
As of January 19, 2024, these warnings equally affected all commercial CAR-T therapies, including Johnson & Johnson’s Carvykti, Novartis’ Kymriah, Bristol Myers Squibb’s Abecma and Breyanzi, and Gilead’s Yescarta and Tecartus.
However, on Tuesday (January 23), the FDA website showed that the warning for Tecartus had been removed, and in a subsequent update, the FDA decided to modify the language in the therapy notification letters, seemingly granting Tecartus some leeway.
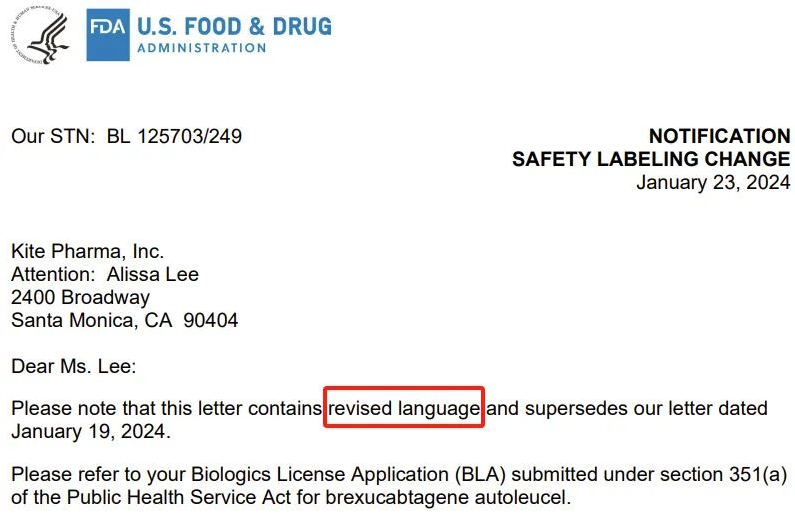
On Tuesday, the FDA wrote to Gilead’s Kite Pharma, stating: “Please note that this letter contains revised wording and supersedes our January 19, 2024, letter.” “For Tecartus (brexucabtagene autoleucel), we have determined that new safety information should be included in the labeling as follows.”
The FDA letter further noted that unlike other BCMA and CD19-directed therapies where T-cell malignancies “have” occurred after treatment, for Tecartus, the same adverse reaction “may” occur.
The FDA’s proposed revisions to the Tecartus safety labeling – which states that T-cell malignancies “have occurred after treatment with BCMA and CD19-directed gene-modified autologous T-cell immunotherapies” and removes “including Tecartus” – indicates that this risk has not yet been observed in Tecartus treatment.
The FDA stated that the new safety information to be included in the labeling is that Tecartus “may increase your risk of getting certain cancers,” including some types of cancers of the immune system.
For example, compared to the letter for Abecma, the Tecartus warning notification is shown in detail below:

Image 2: Tecartus warning notification

Image 3: Abecma warning notification
Content Source:细胞基因研究圈
