Maximum Intention-to-Treat Analysis of Kite Pharma’s Tecartus (Brexucabtagene autoleucel) for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma (R/R MCL)
Maximum Intention-to-Treat Analysis of Kite Pharma’s Tecartus (Brexucabtagene autoleucel) for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma (R/R MCL)
Patients with relapsed/refractory mantle cell lymphoma (R/R MCL) who have failed Bruton’s tyrosine kinase (BTK) inhibitor treatment have a poor prognosis. The ZUMA-2 study found that the CD19 CAR-T cell product Tecartus (Brexucabtagene Autoleucel, brexu-cel) demonstrated favorable efficacy and safety profiles, and other real-world studies have also confirmed its efficacy to be similar to the ZUMA-2 study. However, these studies have analyzed the data from the time of leukapheresis.
Just days apart, Hemasphere and Haematologica published the intention-to-treat (ITT) analysis results of Tecartus treatment for R/R MCL in the UK and France, with sample sizes of 119 and 178 cases, respectively. This article analyzes the latter, while the former is covered in [Hemasphere] Real-world intention-to-treat analysis of brexu-cel for R/R MCL.

Results
This study was based on the French DESCAR-T registry and included 181 patients with R/R MCL (≥1 prior line of immunochemotherapy, including BTK inhibitors) who were approved by a tumor board review (TBR) for Tecartus treatment. Among them, 71.8% did not meet the ZUMA-2 study inclusion criteria, with the most common reasons for ineligibility being the need for bridging therapy other than corticosteroids or BTKi (61.1%), performance status [PS] ≥2 (12%), and a history of malignancy (8.3%). Three patients were excluded because they were still undergoing cell manufacturing at the data cutoff date. Consequently, the “treated group” (received infusion) and “untreated group” (did not receive infusion) comprised 152 and 26 patients, respectively, with the ITT population including 178 patients (Figure 1A). (Note: The ratio of infused to non-infused patients differs significantly from the UK study, where 83 out of 119 approved patients received infusion.)
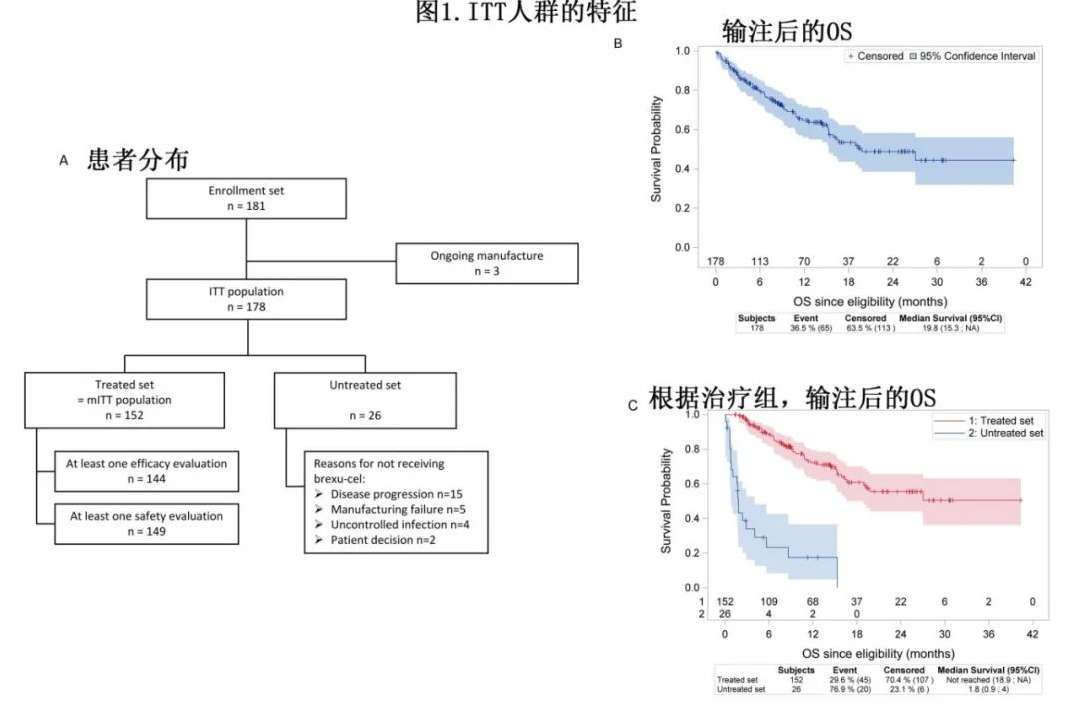
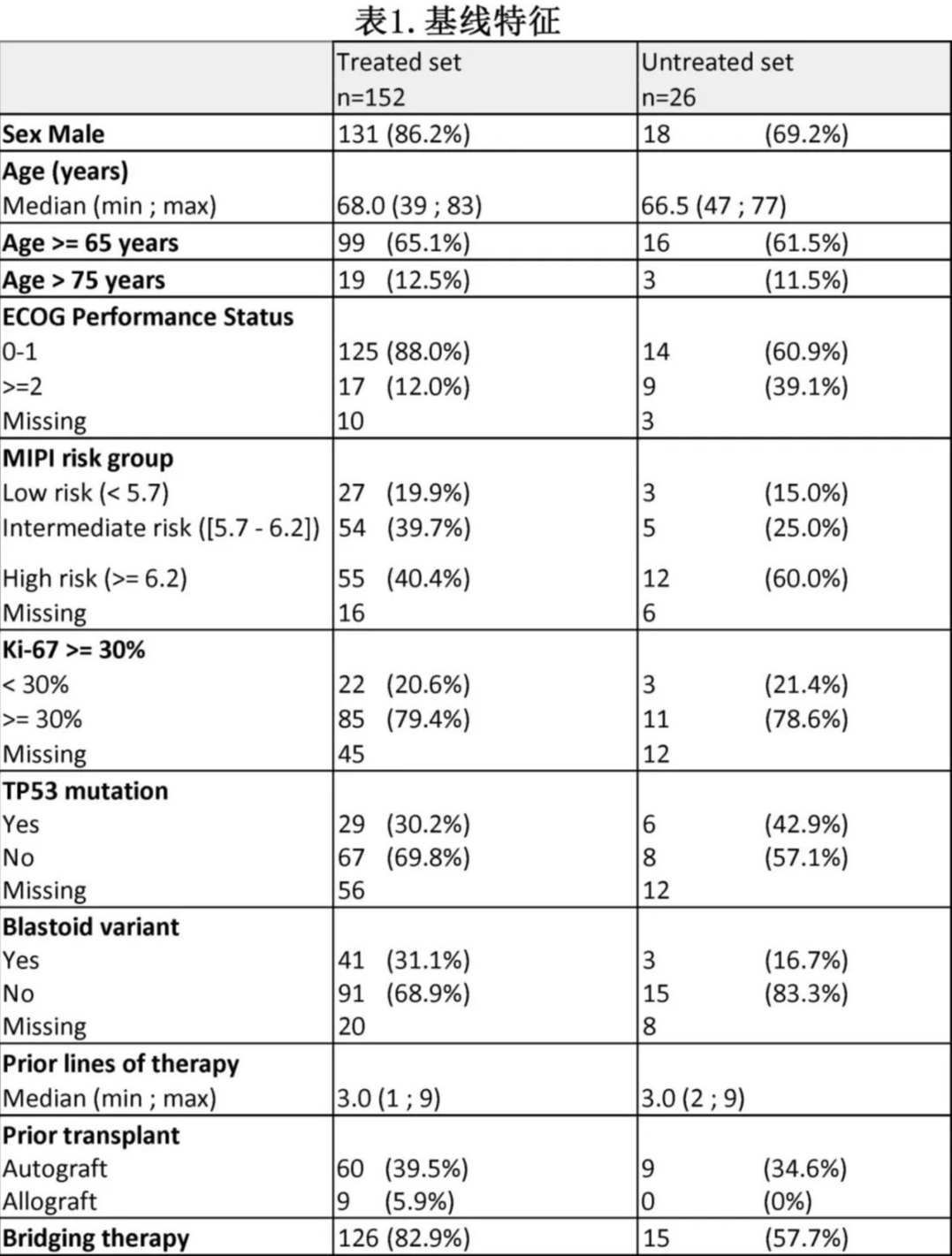
The detailed characteristics of the two groups are shown in Table 1. Among the 152 patients in the “treated group,” 5 had not received BTKi prior to CAR-T treatment, and 2 had not received chemotherapy. The main reasons for not receiving Tecartus in the “untreated group” were disease progression (n=15, with 7 patients dying before infusion) and manufacturing failure (n=5).
In the ITT analysis (n=178) with a median follow-up of 14.2 months, the median overall survival (OS) was 19.8 months (Figure 1B). As expected, the “untreated group” had a poorer OS, with a median of 1.8 months, while the median OS was not reached in the “treated group” (55.6% at 24 months, Figure 1C). The median time from approval to leukapheresis was 20 days, and the median time from leukapheresis to infusion was 39 days. In the “treated group,” 125 patients (82.2%) received bridging therapy, with 61.1% including chemotherapy.
The median follow-up time from the first CAR-T cell infusion (mITT) was 12.2 months. Among the 144 patients with at least one efficacy assessment, the best overall response rate (ORR) was 84.7%, with a complete response (CR) rate of 72.2%. The median progression-free survival (PFS) after infusion was 9.5 months, with a 6-month PFS of 61.3% and a 12-month PFS of 45.6% (Figure 2A). The median OS calculated from infusion was not reached (51.1% at 24 months, Figure 2B). The median duration of CR after infusion was 21.9 months. Among patients with at least one safety assessment (n=149), 87.9% experienced cytokine release syndrome (CRS), and 55% experienced immune effector cell-associated neurotoxicity syndrome (ICANS), with ≥Grade 3 CRS or ICANS in 12.1% (n=18) and 15.4% (n=23) of patients, respectively.
The median time to CRS onset was 5 days, with a median CRS duration of 6 days; the median time to ICANS onset was 7 days, with a median ICANS duration of 7 days. Medications used to manage CRS and/or ICANS included tocilizumab (74.8%), corticosteroids (64.9%), anakinra (11.5%), and siltuximab (5.3%, all in combination with tocilizumab).
At 3 months, 19.7% (n=24) of evaluable patients experienced any-grade persistent cytopenias, with 13 and 1 patients experiencing ≥Grade 3 neutropenia and thrombocytopenia, respectively. From infusion to Day 10, 25.5% of patients (n=38) experienced ≥Grade 3 infections, mostly bacterial (n=25, 16.8%). Overall, 34.3% of patients (n=46) required intensive care unit (ICU) transfer, with a median hospital stay of 6 days, primarily due to CRS (n=44: Grade 2 in 26 cases, ≥Grade 3 in 18 cases) and/or ICANS (n=36: Grade 2 in 13 cases, ≥Grade 3 in 23 cases). All patients recovered from the ICU, except for 2 cases of Grade 5 CRS.
Among the 152 infused patients, 46 deaths occurred, with a non-relapse mortality rate of 11.2%. The primary causes of death were progressive disease (n=29), followed by infectious events (n=11: bacterial sepsis in 7 cases, COVID-19 pneumonia in 3 cases, and toxoplasmosis in 1 case), CRS (n=2), myelodysplastic syndrome (MDS) (n=2), and unexplained deaths (n=2). Before enrollment, 9 infused patients had received allogeneic stem cell transplantation, and none developed graft-versus-host disease (GVHD).
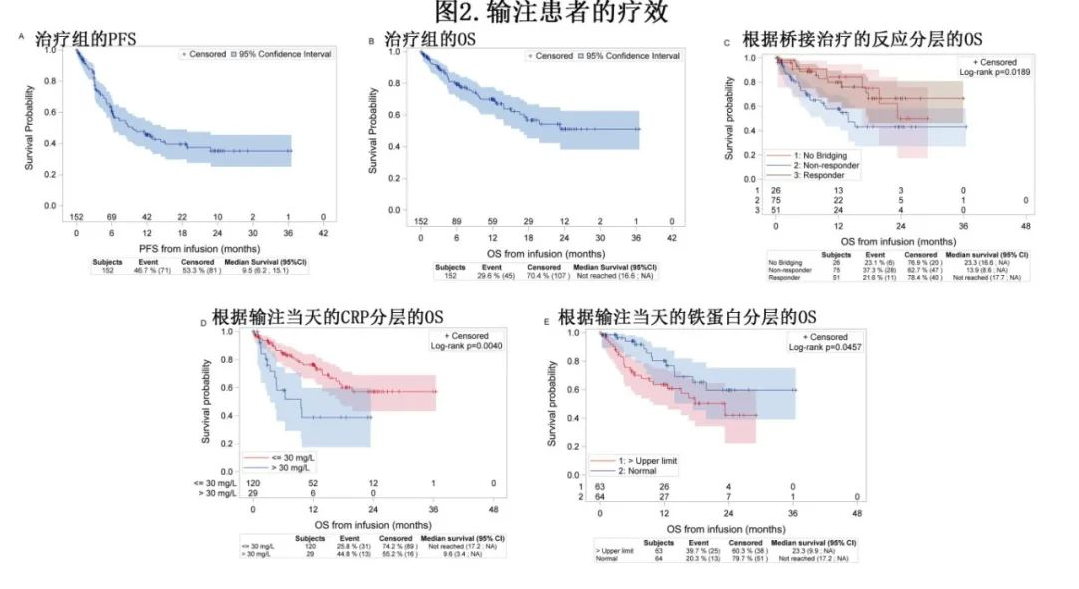
The authors also conducted pre-planned exploratory analyses. The need for bridging therapy and response to bridging therapy were significantly associated with post-infusion OS. The 12-month OS rate was 58% for patients who received bridging therapy without a response, 79.9% for patients with a response, and 84.3% for patients who did not require bridging therapy (Figure 2C). At the time of the first infusion, CRP >30 mg/L and ferritin >upper limit of normal (ULN) were significantly associated with shorter OS (p=0.004 and p=0.04, respectively; Figures 2D and 2E). There were no differences in OS or PFS based on bridging time, age, or LDH level at infusion.
Summary
This “real-world” experience supports the use of Tecartus in patients with R/R MCL progressing after BTKi, particularly when the disease is controllable prior to infusion.
References
Herbaux C, et al. Brexucabtagene autoleucel in relapsed or refractory mantle cell lymphoma, intention-to-treat use in the DESCAR-T registry.Haematologica . 2024 Jun 20. doi: 10.3324/haematol.2023.284786.
Content Source:聊聊血液
