Real-World Experience Comparing Kymriah and Yescarta for the Treatment of Relapsed/Refractory Large B-Cell Lymphoma (R/R LBCL) in 307 Cases
Real-World Experience Comparing Kymriah and Yescarta for the Treatment of Relapsed/Refractory Large B-Cell Lymphoma (R/R LBCL) in 307 Cases
Several CAR-T products have been approved for the treatment of large B-cell lymphoma. However, the pivotal studies of different products have significant differences in terms of patient characteristics, vein-to-vein time, lymphodepletion, bridging chemotherapy, and outcomes. Additionally, the real-world results post-approval deviate from the pivotal studies to some extent. Furthermore, there is a lack of head-to-head comparisons between different products.
Axicabtagene ciloleucel (axi-cel, Yescarta) and tisagenlecleucel (tisa-cel, Kymriah) were the first two CAR-T products approved in Europe for the treatment of relapsed/refractory large B-cell lymphoma (R/R LBCL) patients, and they have accumulated some real-world experience. To compare the efficacy and safety of Yescarta and Kymriah in the real-world setting, Spanish researchers conducted a multicenter, retrospective study in 307 R/R LBCL patients. The results showed that compared to patients treated with Kymriah, patients treated with Yescarta experienced more toxicity, but had a similar non-relapse mortality rate. Additionally, the efficacy was not significantly different between the two groups. The results have been published in the journal Haematologica.

Study Design
The authors retrospectively collected data from all consecutive patients who underwent leukapheresis (LP) and received Yescarta or Kymriah at 12 institutions in Spain between November 2018 and August 2021. Survival outcomes were evaluated in all patients who underwent LP (intent-to-treat, ITT) and in those who received CAR-T cell infusion.
Lymphodepletion (LD) consisted of 3 consecutive days of fludarabine (30 mg/m2 for Yescarta, 25 mg/m2 for Kymriah) and cyclophosphamide (500 mg/m2 for Yescarta, 250 mg/m2 for Kymriah). 2-4 days after LD, patients received CAR-T cell infusion as inpatients to ensure close monitoring for adverse events. Grading of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) followed the American Society for Transplantation and Cellular Therapy (ASTCT) recommendations, and treatment followed local institutional guidelines based on national guidelines.
Study Results
Patient Characteristics
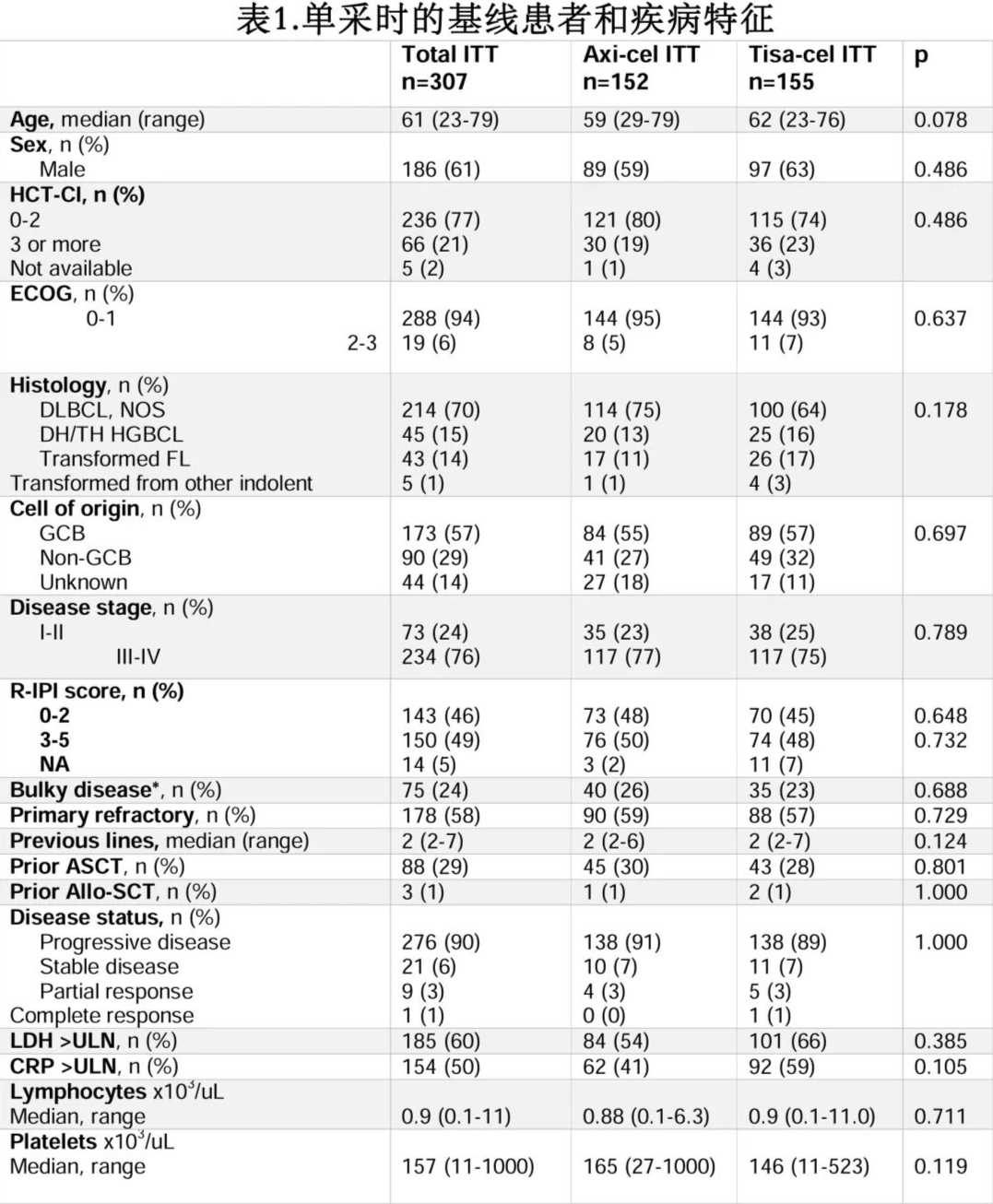
Of the 307 R/R LBCL patients who underwent LP, 152 received Yescarta, and 155 received Kymriah. Of these, 261 (85%) received CAR-T cell infusion (n=134, 88% and n=127, 82%, respectively). The main reasons for not receiving infusion were disease progression (Yescarta group n=12, 66%; Kymriah group n=25, 89%). The median time from LP to infusion was 41 days for the Yescarta group and 52 days for the Kymriah group (p=0.006).
Patient and disease characteristics at LP are shown in Table 1. The median age was 61 years. The most common subtypes were diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS) (70%), followed by high-grade B-cell lymphoma (15%) and transformed follicular lymphoma (14%). Patient and disease characteristics at LP were similar between the Yescarta and Kymriah groups (Table 1). Among the infused patients, 210/261 (80%) received bridging therapy (BT) before infusion, mostly chemotherapy-containing regimens (n=127, 60%). The proportion of patients receiving BT before Yescarta and Kymriah was similar (78% vs. 83%). Thirty (14%) patients achieved a response to BT (21 partial responses [PR], 9 complete responses [CR]), mostly after chemotherapy. Baseline characteristics at LD were similar between patients receiving Yescarta and Kymriah. The median follow-up from infusion was 8.2 months for the Yescarta group and 12.4 months for the Kymriah group.
Safety
CRS and ICANS: Among all infused patients, 211 (81%) and 78 (30%) experienced any-grade CRS and ICANS, respectively; the median time to CRS onset was 2 days, and the median time to ICANS onset was 7 days (Table 2).
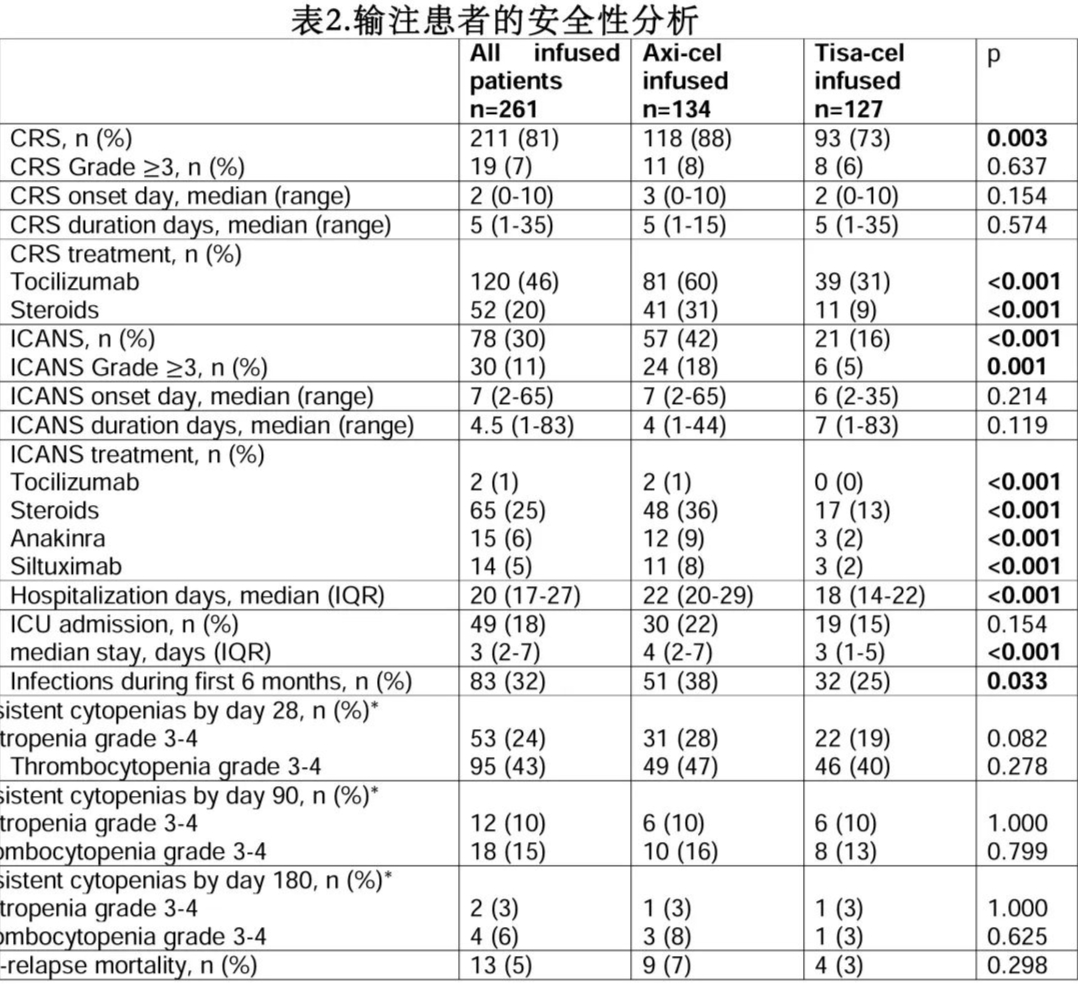
The incidence of any-grade CRS was higher in the Yescarta group than the Kymriah group (88% vs. 73%, p=0.003), but the incidence of grade ≥3 CRS was similar (8% vs. 6%, p=0.637). The incidence of any-grade and grade ≥3 ICANS was significantly higher in the Yescarta group (42% vs. 16%, p<0.001, and 18% vs. 5%, p=0.001, respectively). The time to onset and duration of CRS and ICANS were similar between Yescarta and Kymriah (Table 2). Additionally, 4 patients developed macrophage activation syndrome (MAS), 1 in the Yescarta group and 3 in the Kymriah group (1 fatal).
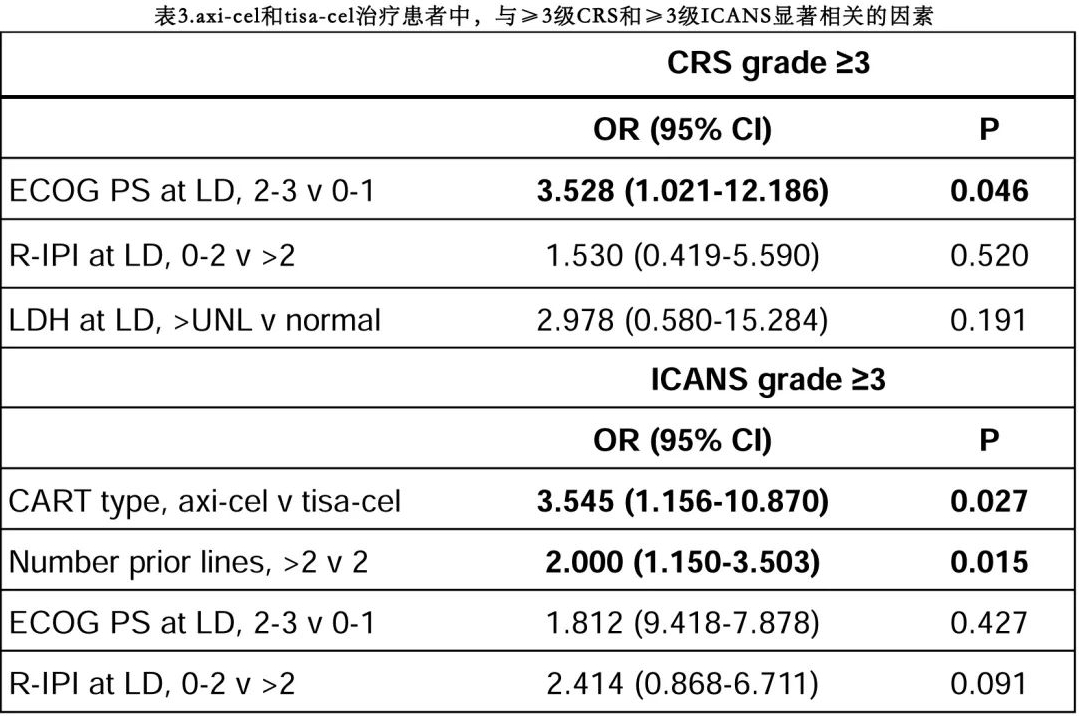
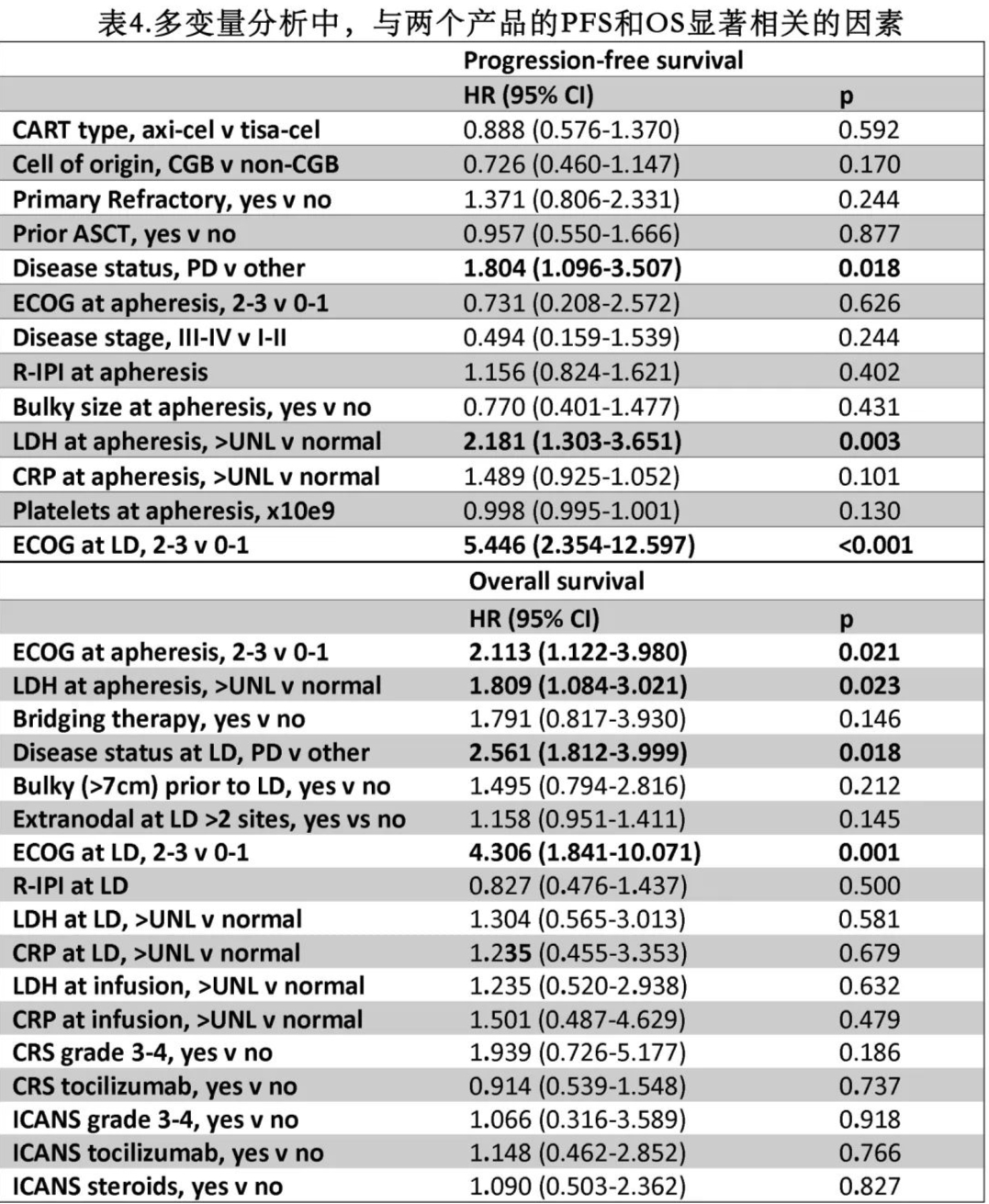
In multivariate analysis, an ECOG performance status (PS) ≥2 at LD was the only factor associated with an increased risk of grade ≥3 CRS (p=0.046), while the use of Yescarta (p=0.027) and having received >2 prior lines of therapy (p=0.015) were associated with an increased risk of grade ≥3 ICANS (Table 3).
Hematological Toxicity and Infections: Among 220 evaluable patients, 53 (24%) and 95 (43%) experienced grade 3-4 neutropenia and thrombocytopenia, respectively, within 1 month of infusion. Among 123 evaluable patients at 3 months, 12 (10%) and 18 (15%) had persistent cytopenias. The rates of persistent cytopenias were not significantly different between the Yescarta and Kymriah groups (Table 2).
Eighty-three (32%) infused patients experienced 91 infection events within the first 6 months after CAR-T cell infusion, mainly bacterial infections (n=54, 59%), followed by viral infections (n=31, 34%) and fungal infections (n=6, 7%). Six patients experienced human herpesvirus 6 reactivation, all after Yescarta infusion. Two patients experienced COVID-19 infection within the first 6 months after infusion, with one fatality. Notably, 3 additional patients died from COVID-19 infection after 6 months. The infection rate within the first 6 months after infusion was higher in the Yescarta group (Table 2).
Hospitalizations, ICU Admissions, and Non-Relapse Mortality: The median length of hospitalization was 22 days for the Yescarta group and 18 days for the Kymriah group (p<0.001). Twenty-two percent of patients in the Yescarta group and 15% in the Kymriah group required ICU admission (p=0.154), with a median ICU stay of 4 and 3 days, respectively (p<0.001). The non-relapse mortality rate was 5% among all infused patients and was similar between the two groups (p=0.298). Nine patients (7%) died in the Yescarta group due to infections (2 bacterial, 1 COVID-19, 1 fungal, 1 unspecified), ICANS (2 cases), CRS (1 case), and tumor lysis syndrome (1 case), while 5 patients (4%) died in the Kymriah group due to COVID-19 infection (2 cases), CRS (1 case), ICANS (1 case), and MAS (1 case).
Efficacy
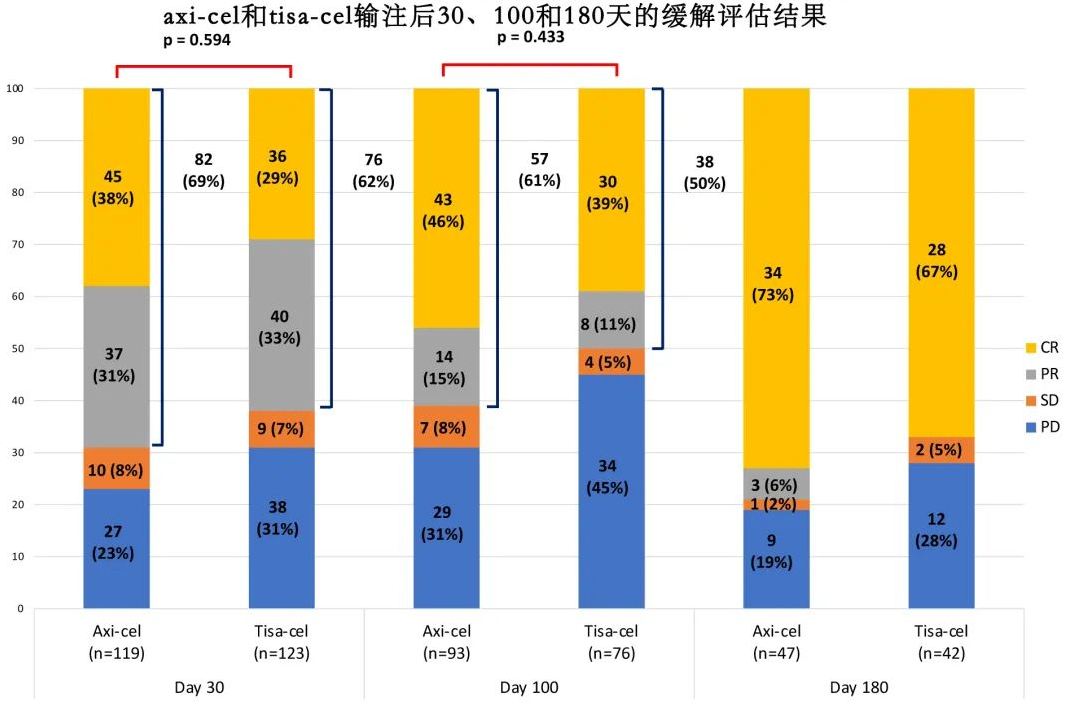
Response Rates: The overall response rate (ORR) among all infused patients was 57% (38% CR, 19% PR). The median duration of response (DOR) among all infused patients was 14.1 months, and the median DOR was not reached in patients achieving CR. The ORR in the Yescarta group was 60% (42% CR, 18% PR), with a median DOR of 12.5 months. In the Kymriah group, the ORR was 54% (n=68) (34% CR, 19% PR), with a median DOR of 14.1 months. The median DOR was not significantly different between the two groups (p=0.494).
Progression-Free Survival (PFS) and Overall Survival (OS): In the ITT analysis, with a median follow-up of 9.2 months, the median PFS and OS were 4.8 months and 11.7 months, respectively. The estimated 12-month PFS and OS rates were 34% and 48%, respectively (Figure 1). The 12-month PFS and OS rates for patients intended to receive Yescarta and Kymriah were 41% and 27% (p=0.091), and 50% and 45% (p=0.07), respectively.
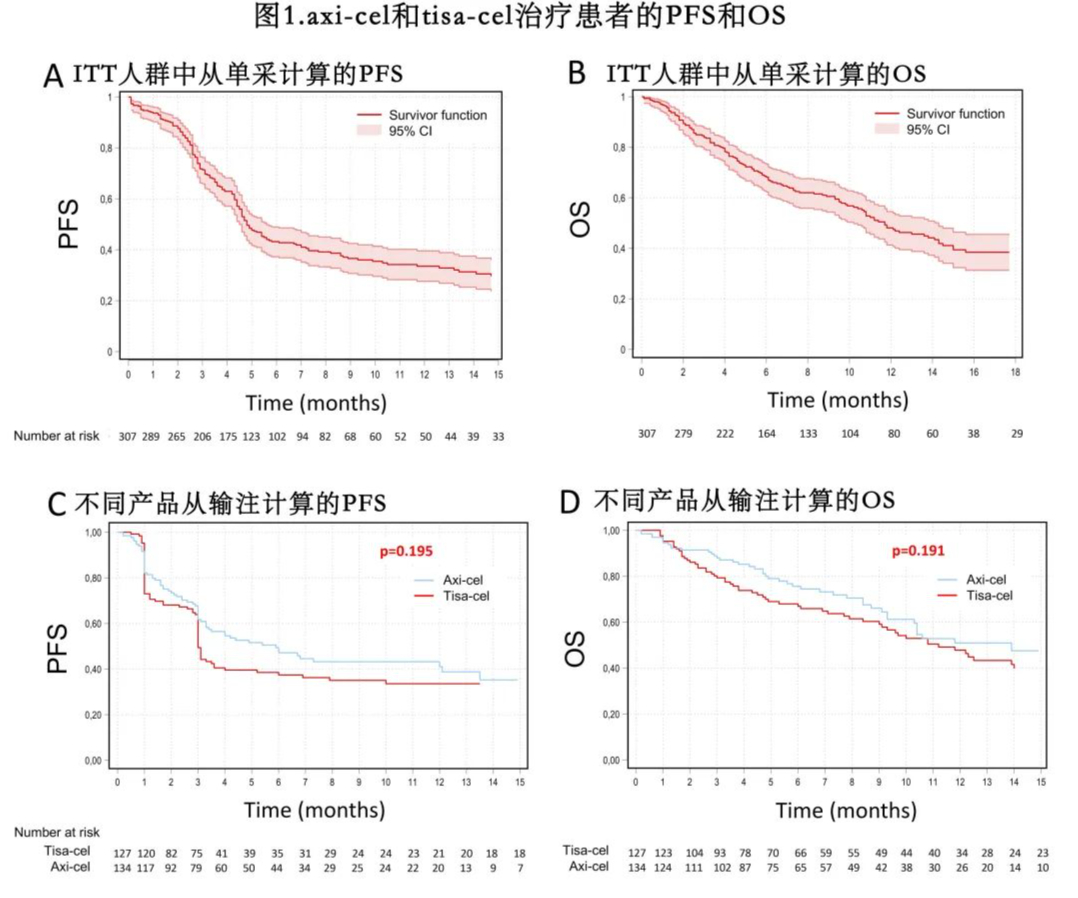
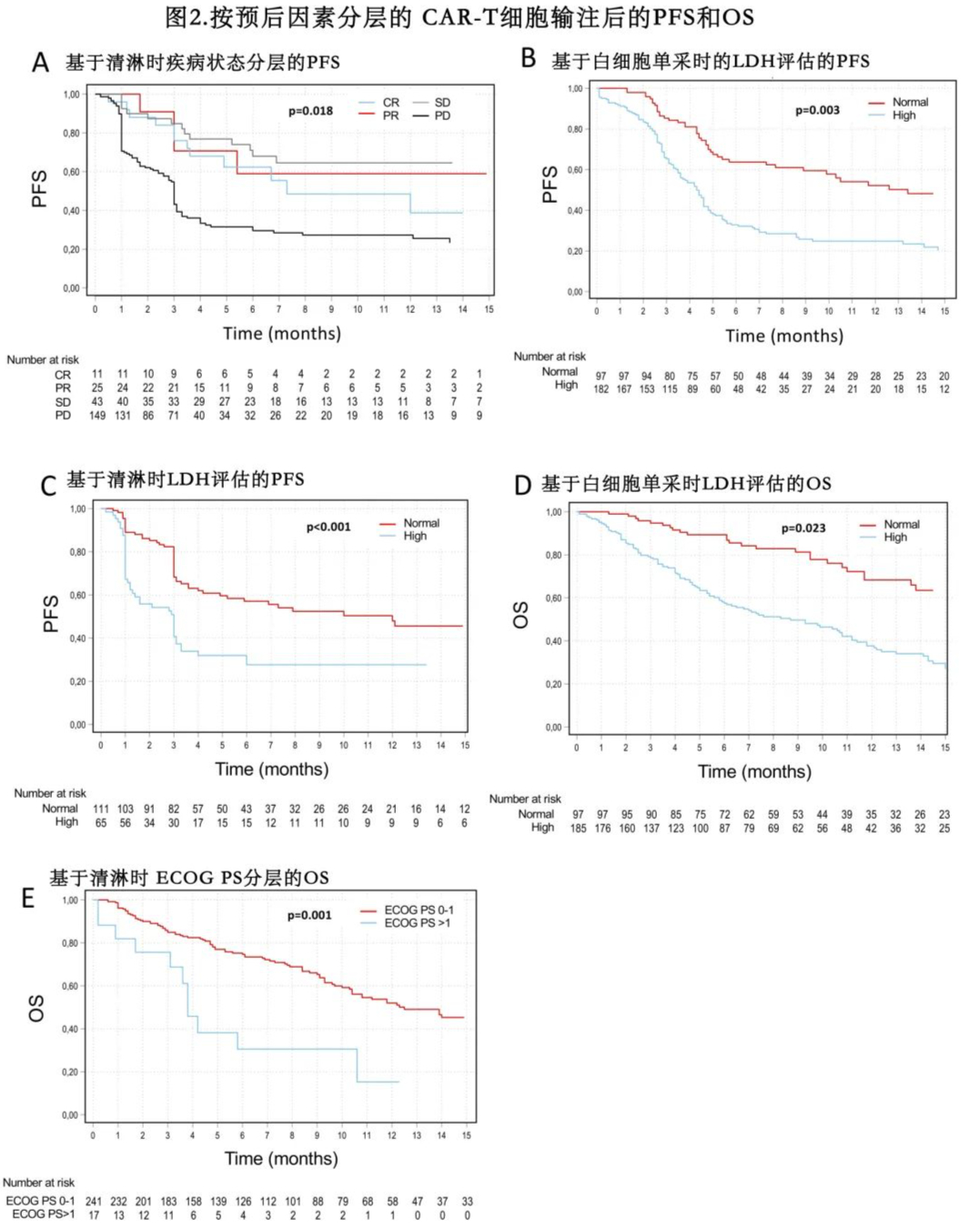
The median PFS was 5.9 months for the Yescarta group and 3.0 months for the Kymriah group, while the median OS was 13.9 months and 11.2 months, respectively (Figure 1). The estimated 12-month PFS rates were 41% and 33% (p=0.195), and the 12-month OS rates were 51% and 47% (p=0.191) for the Yescarta and Kymriah groups, respectively. In multivariate analysis, elevated lactate dehydrogenase (LDH) at LP (p=0.003), ECOG PS ≥2 at LD (p<0.001), and progressive disease at LD (p=0.018) were independently associated with worse PFS (Table 4 and Figure 2). Factors independently associated with worse OS included elevated LDH at LP (p=0.023), ECOG PS ≥2 at LP (p=0.021), progressive disease at LP (p=0.018), and ECOG PS ≥2 at LD (p=0.001). Additionally, patients with LDH >2× upper limit of normal (ULN) had worse PFS (HR=2.5, p<0.001) and OS (HR=2.1, p<0.001) compared to those with LDH 1-2× ULN.
Discussion
In this study, patient and disease characteristics at LP were similar between the two cohorts, suggesting that CAR-T product selection may have been driven by other factors, including logistics, manufacturing slot availability, and anticipated turnaround time. The higher proportion of patients receiving Yescarta infusion may have been influenced by the shorter turnaround time. Although potential unintended biases in product selection have not been established, the similarity in patient characteristics between the two groups allows for a comparison of outcomes after Yescarta and Kymriah treatment.
Overall, the real-world safety and efficacy results in this experience were comparable to those reported in the pivotal trials. Patients treated with Yescarta experienced higher toxicity but had a similar non-relapse mortality rate compared to those treated with Kymriah, and the efficacy of the two products was similar.
References
Mi Kwon,et al.Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma.Haematologica . 2022 Jun 30. doi: 10.3324/haematol.2022.280805.
Content Source:聊聊血液
