Successful Case Analysis of CAR-T for Multiple Myeloma
Successful Case Analysis of CAR-T for Multiple Myeloma
Professor Jin Jie’s team from the Department of Hematology at the First Affiliated Hospital of Zhejiang University School of Medicine applied the CAR-T cell therapy Zevorcabtagene Autoleucel (CT053) to treat a high-risk multiple myeloma patient who experienced early relapse after anti-CD38 monoclonal antibody treatment. After receiving Zevorcabtagene Autoleucel treatment, the patient achieved a stringent complete response, and subsequent follow-up showed a progression-free survival (PFS) of over 60 months. This article summarizes the diagnosis and treatment process of this case and the experience with the medication for reference.
Case Introduction
Multiple myeloma (MM) is a malignant disease characterized by clonal proliferation of abnormal plasma cells. In many countries, it is the second most common hematological malignancy, predominantly affecting the elderly population, and currently remains incurable. With the continuous introduction of new drugs and improved testing methods, the diagnosis and treatment of multiple myeloma have been continuously improving1. However, due to the high heterogeneity of multiple myeloma, high-risk patients benefit very little from new-generation immunomodulatory drugs, new-generation proteasome inhibitors, anti-CD38 monoclonal antibodies, bispecific antibodies, antibody-drug conjugates (ADCs), and autologous stem cell transplantation (ASCT). In particular, patients with functional high risk (FHR, poor response to induction therapy or early relapse) and ultra-high-risk cytogenetic abnormalities (presence of ≥2 high-risk cytogenetic abnormalities) have a worse prognosis2-3. Improving the prognosis of these high-risk patients is one of the major challenges in current multiple myeloma clinical practice, and CAR-T cell therapy may bring new breakthroughs and developments for the treatment of these high-risk MM patients.
In March 2018, our hospital admitted a high-risk multiple myeloma patient who experienced early relapse after anti-CD38 monoclonal antibody treatment. After receiving Zevorcabtagene Autoleucel (CT053) CAR-T cell therapy, the patient achieved a stringent complete response, and subsequent follow-up showed a progression-free survival (PFS) of over 60 months.
Case data
General Information: The patient is a 40-year-old married Han Chinese female who has been diagnosed with multiple myeloma for over 3 years. Due to poor response to the third-line anti-CD38 targeted therapy, with disease progression occurring within just 6 months, BCMA CAR-T cell therapy was considered. The patient visited our outpatient department seeking CAR-T cell treatment and was admitted to the hospital on March 19, 2018, with a proposed diagnosis of “multiple myeloma.”
History of Present Illness: More than 3 years ago (December 2014), the patient developed lumbago and back pain without any apparent cause. The pain was initially tolerable but gradually worsened, making it impossible for the patient to walk. There were no associated symptoms such as chest tightness, chest pain, abdominal pain, diarrhea, dizziness, or headache. The patient sought medical attention at another hospital.
1) Complete Blood Count – C-Reactive Protein (CRP): White Blood Cells (WBC) 4.1×109/L, Hemoglobin (HGB) 96 g/L, Platelets (PLT) 144×109/L, CRP 1.32 mg/L.
2) Blood Biochemistry: Total Protein 163.2 g/L, Albumin 32.1 g/L, Globulin 131.1 g/L.
3) Immunoglobulins: IgG 92.55 g/L, IgM 0.19 g/L, IgA 0.006 g/L.
4) Lumbar Spine MRI: Diffuse bone disease.
5) Serum Immunofixation Electrophoresis: IgG-λ positive, markedly elevated serum free light chain ratio (λ/κ).
6) Bone Marrow Smear: Increased plasma cells, with binucleated and trinucleated plasma cells present.
7) Flow Cytometry Immunophenotyping: Monoclonal proliferation of plasma cells accounting for 10.74% of nucleated cells in the bone marrow, suggestive of plasma cell myeloma.
Immunophenotypic Characteristics: Strongly positive for CD38, CD138, CD56; CD38-positive cells were λ-positive and κ-negative. Karyotype: 46,XX[20]. Multiple myeloma was the primary consideration.
Subsequent Treatment:
2014.12-2015.1 (First-line Induction Therapy): The patient received 2 cycles of PAD (Bortezomib + Doxorubicin + Dexamethasone) regimen. She reported significant numbness and tingling in the limbs after chemotherapy.
2015.2-2015.6 (First-line Induction Therapy): The patient then received 1 cycle of VAD (Vincristine + Doxorubicin + Dexamethasone) regimen, 2 cycles of TCD (Thalidomide + Cyclophosphamide + Dexamethasone) regimen, and 2 cycles of RD (Lenalidomide + Dexamethasone) regimen.
In March 2015, a review showed IgG 8.71 g/L, and serum immunofixation electrophoresis was positive for IgG-λ.
2015.11-2015.12 (First-line Autologous Transplant): In November 2015, the patient received consolidation therapy at a local hospital.
1) Complete Blood Count: White Blood Cells (WBC) 5.19×109/L, Hemoglobin (HGB) 122 g/L, Platelets (PLT) 190×109/L.
2) Blood Biochemistry: Prealbumin 412.1 mg/L.
3) Immunoglobulins: IgG 11.34 g/L, IgA 0.06 g/L.
4) CT scan: Multiple osteolytic bone destruction in the thoracic vertebrae.
The patient then underwent autologous stem cell transplantation (pre-transplant assessment: Partial Response [PR]). On November 10, 2015, melphalan was administered for conditioning, and peripheral blood stem cells were reinfused on November 12, 2015, with supportive therapy including blood components, G-CSF, and TPO for stimulating hematopoiesis. Post-transplant, the patient developed liver dysfunction and gastrointestinal adverse reactions. On the 30th day post-transplant, she experienced cholecystitis, which resolved with treatment.
2015.12-2016.9 (First-line Maintenance Therapy): Following the transplant, the patient received maintenance therapy, with regimens including TD (Thalidomide + Dexamethasone) and TCD (Thalidomide + Cyclophosphamide + Dexamethasone).
March 2016 Review:
1) Immunoglobulins: IgG 15.35 g/L
2) Serum Immunofixation Electrophoresis: Positive for IgG-λ
3) Bone Marrow Smear: Slightly increased immature cells in some areas
2016.9-2017.5 (Second-line Therapy): In September 2016, the patient received treatment with IFN+RD (Interferon + Lenalidomide + Dexamethasone) regimen.
October 2016 Review:
1) Immunoglobulins: IgG 10.30 g/L
2) Serum Immunofixation Electrophoresis: Positive for IgG-λ
May 2017 Review at Local Hospital:
1) Immunoglobulins: IgG 15.9 g/L, IgA 0.08 g/L, IgM 0.54 g/L
2) Erythrocyte Sedimentation Rate (ESR): 41.00 mm/h
2017.5-2018.1 (Third-line Anti-CD38 Monoclonal Antibody Therapy): Subsequently, the patient’s disease progressed, and she was enrolled in a clinical trial of anti-CD38 monoclonal antibody for further treatment. However, the patient had a poor response to the anti-CD38 antibody, with disease progression occurring within just 6 months, leading to discontinuation from the clinical trial.
2018.3-2018.4 (Fourth-line CAR-T Therapy): After the anti-CD38 antibody treatment, the patient’s condition progressed again. The patient visited our outpatient department seeking CAR-T therapy and was admitted to the hospital with a proposed diagnosis of “multiple myeloma.” Details of the previous diagnosis and treatment are shown in Table 2-1. Since the onset of the disease, the patient has remained alert and has maintained a good appetite, sleep, and bowel movements, although urination has been reduced. The patient’s weight has increased by 15 kg.
Table 2-1. early diagnosis and treatment process
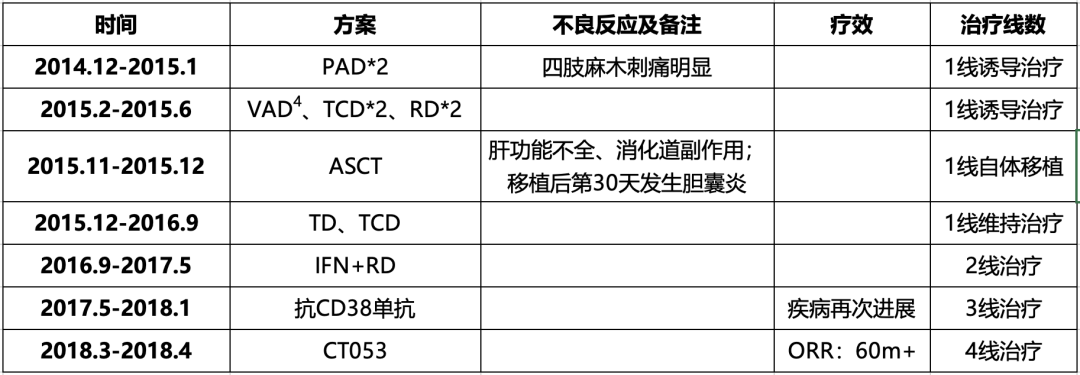
1early diagnosis and treatment process
P:Bortezomib;A:Pharmorubicin;D:Dexamethasone;T:Thalidomide;C:Cyclophosphamide;R:Lenalidomide;V:Vindesine;ASCT:Autologous Stem Cell Transplan-Tation;IFN:Interferon;CT053:Zevorcabtagene Autoleucel。
Auxiliary Examinations:
1) Immunoglobulins IgG: 92.55 g/L (2014.12), 8.71 g/L (2015.3.3), 11.34 g/L (2015.11.5), 15.35 g/L (2016.3.2), 10.30 g/L (2016.10.25), 15.90 g/L (2017.5.11).
2) Serum Immunofixation Electrophoresis: Positive for IgG-λ (2014.12), Positive for IgG-λ (2015.3.3), Positive for IgG-λ (2016.3.2), Positive for IgG-λ (2016.10.25).
Clinical Diagnosis: Multiple Myeloma (IgG-λ type, Durie-Salmon Stage IIIA, International Staging System Stage IIA).
Treatment Process:
As the patient had a poor response to the anti-CD38 targeted therapy, with disease progression occurring within just 6 months, BCMA CAR-T therapy was considered.
In March 2018, the patient successfully underwent leukapheresis to obtain a sufficient quantity of T lymphocytes. In April 2018, after lymphodepleting conditioning, the patient received an infusion of 1.5 × 10^8 CAR BCMA-positive T cells (Zevorcabtagene Autoleucel). The lymphocyte monitoring is shown in Figure 3-1, with lymphocyte levels recovering to normal levels on the 9th day post-infusion. Temperature monitoring is shown in Figure 3-2: the highest temperature on the day of infusion was 37.4°C, but the patient developed a fever starting on the first day after infusion, with a maximum temperature of 38.5°C lasting for 9 days. Inflammatory markers such as CRP were slightly elevated, showing a gradual upward trend (2.96-48.58 mg/L). On the first day after infusion, considering the patient’s symptoms of nasal congestion and sore throat, an acute upper respiratory tract infection was suspected, and piperacillin/tazobactam and moxifloxacin were administered for anti-infective treatment. On the 10th day post-infusion, the patient’s temperature returned to normal, with no further fever episodes, and the CRP level was 4.78 mg/L on the 13th day post-infusion.
Additionally, CAR-T cell monitoring showed a rapid in vivo expansion, peaking on the 11th day, with a peak value of 38,988 CAR-BCMA copies/μg genomic DNA (see Figure 3-3). The persistence duration was 8 weeks (2 months), after which the CAR-BCMA copies fell below the detectable limit. However, remission persisted, and subsequent follow-up showed long-term disease control, with a progression-free survival exceeding 60 months. The patient did not experience Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) or Grade ≥3 Cytokine Release Syndrome (CRS) post-infusion, only a transient elevation of IL-6 that resolved without treatment by the next day.
After evaluation, the patient was discharged, and subsequent follow-up showed achievement of stringent complete remission, with the 2016 IMWG MRD assessment indicating next-generation flow cytometry-negative MRD status.
Figure 3-1. lymphocyte monitoring
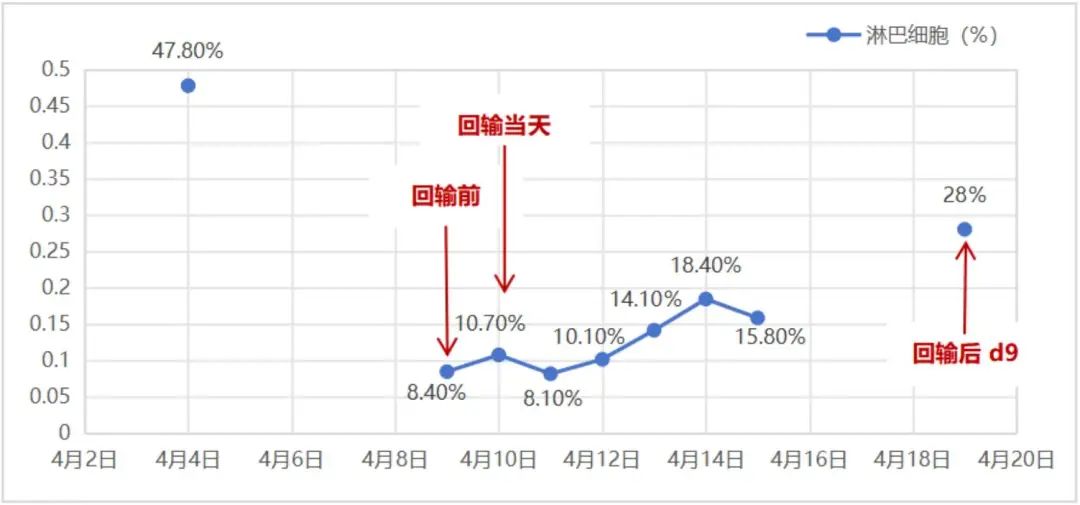
lymphocyte monitoring
Figure 3-2. temperature monitoring (April 3-23, 2018)
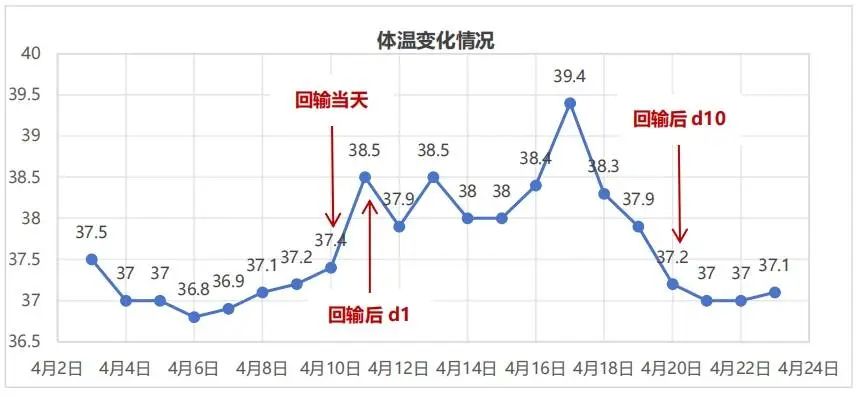
temperature monitoring
Figure 3-3. Car-t cell expansion monitoring (from the day of reinfusion to the 112th day after reinfusion)
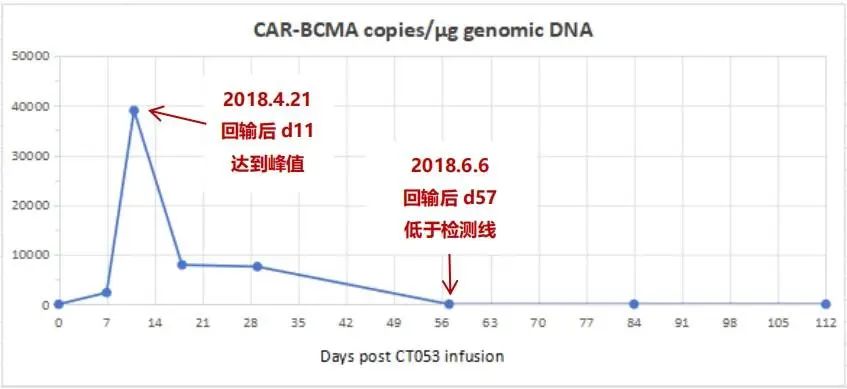
4Car-t cell expansion monitoring
Analysis And Discussion
In 2016, Ali et al.5 reported the first global study of anti-BCMA CAR-T cell therapy for relapsed/refractory multiple myeloma, demonstrating its efficacy and safety. Subsequently, numerous clinical studies on anti-BCMA CAR-T cell therapy for multiple myeloma have been conducted globally. Currently, the overall response rates range from 73% to 100%, with some studies reporting stringent complete response/complete response rates exceeding 50%. The CAR-T cell therapy used in this case was Zevorcabtagene Autoleucel (CT053). The 2022 Chinese CAR-T Consensus shows that its sCR/CR rate is 79%, which is outstanding among similar therapies. Additionally, the median progression-free survival (mPFS) for Zevorcabtagene Autoleucel is 22.7 months6, and the latest 3-year follow-up data presented at ASH 2023 showed an mPFS of 25 months7. In terms of mPFS, Zevorcabtagene Autoleucel appears to perform better based on the data.
Excluding the similar incidence of Cytokine Release Syndrome (CRS) compared to CAR-T therapy for lymphoma, over 80% of patients receiving BCMA CAR-T cell therapy experience Grade 3 or higher hematological toxicity. However, the incidence of severe CRS and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) is lower than in other B-cell malignancies. In this case, the patient did not experience ICANS or severe CRS after receiving Zevorcabtagene Autoleucel, demonstrating good safety.
Table 4-1. clinical studies of BCMA car-t cells in the treatment of multiple myeloma at home and abroad
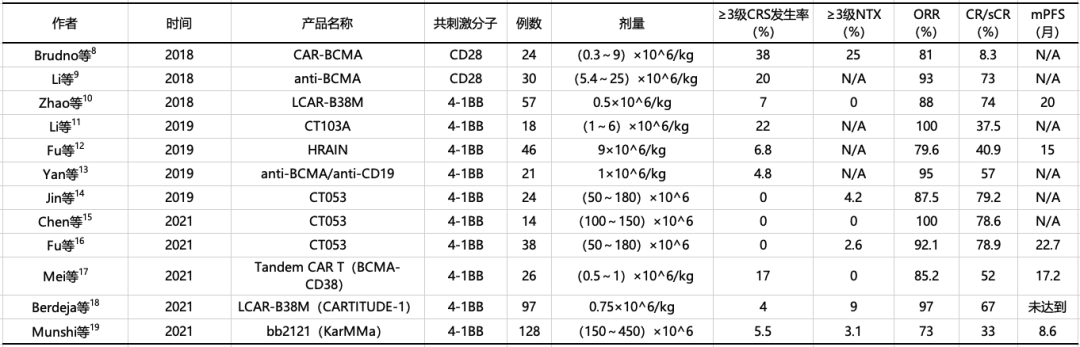
5clinical studies of BCMA car-t cells in the treatment of multiple myeloma at home and abroad
Note: BCMA: B-cell Maturation Antigen; CAR-T cells: Chimeric Antigen Receptor T cells; CRS: Cytokine Release Syndrome; NTX: Neurotoxicity; ORR: Overall Response Rate; sCR/CR: Stringent Complete Response/Complete Response; PFS: Progression-Free Survival; N/A: Not Reported.
Currently, commonly used lymphodepleting regimens include cyclophosphamide combined with fludarabine or cyclophosphamide alone. However, the recommended standard regimen is fludarabine (25 mg/m2, days -5 to -3) in combination with cyclophosphamide (300 mg/m2, days -5 to -3), as the lymphodepletion regimen is closely related to the ability to achieve better treatment responses and sustained remission20. Among the clinical studies of various BCMA CAR-T products, only CT053 and HRAIN have adopted the standard fludarabine (25 mg/m2, days -5 to -3) and cyclophosphamide (300 mg/m2, days -5 to -3) lymphodepletion regimen. Although the FDA-approved LCAR-B38M and bb2121 CAR-T products use the same dose of cyclophosphamide, the fludarabine dose is relatively higher (30 mg/m2, days -5 to -3), as shown in Table 4-2. In this case, the standard cyclophosphamide and fludarabine lymphodepletion regimen was used, with good lymphodepletion efficacy.
Table 4-2. pretreatment schemes for some clinical studies of BCMA car-t cells in the treatment of multiple myeloma at home and abroad
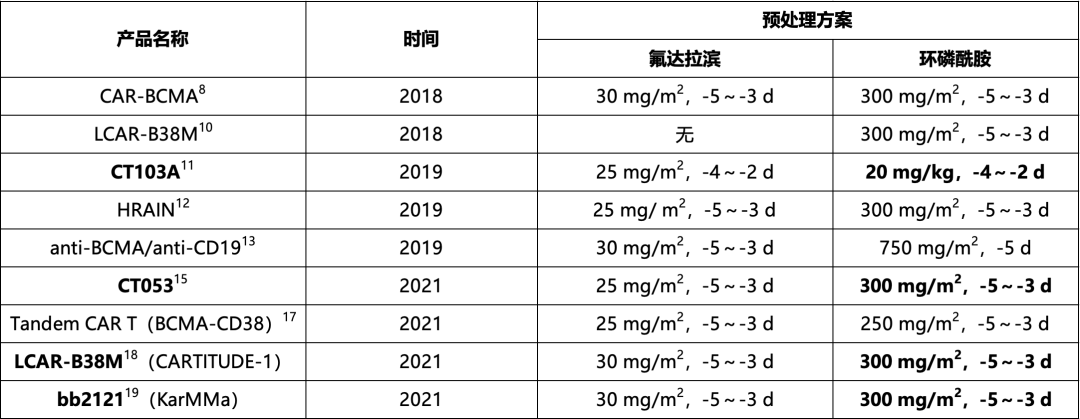
pretreatment schemes for some clinical studies of BCMA car-t cells in the treatment of multiple myeloma at home and abroad
Note: BCMA: B-cell Maturation Antigen; CAR-T cells: Chimeric Antigen Receptor T cells.
Regarding the factors influencing sustained remission after CAR-T cell therapy, a review by Cappell KM et al. published in 2023 in Nature Reviews Clinical Oncology (IF=78.8)20 shows that the efficacy of CAR-T cell therapy (including initial treatment response and sustained remission) is somewhat correlated with the peak level of CAR-T cells in the CAR-T cell kinetics, but does not appear to be related to the persistence duration of CAR-T cells in vivo. In this case, although the CAR-BCMA copies were undetectable after 8 weeks, the patient remained in remission, with a total remission duration exceeding 60 months (260+ weeks). Apart from the peak CAR-T cell level in cell kinetics, sustained remission is closely related to the depth of treatment response, type of hematological malignancy, tumor size and location, and lymphodepletion regimen.
Zevorcabtagene Autoleucel is a fully human BCMA-targeted CAR-T product developed domestically in China. Currently, based on relevant IIT, Phase I, and Phase II clinical data, Zevorcabtagene Autoleucel has demonstrated outstanding efficacy and safety, with significant advantages over similar products.
1) Zevorcabtagene Autoleucel leads to durable remissions, with a 12-month PFS rate of 85.7% and a remarkable median PFS of 25 months, outperforming similar products.
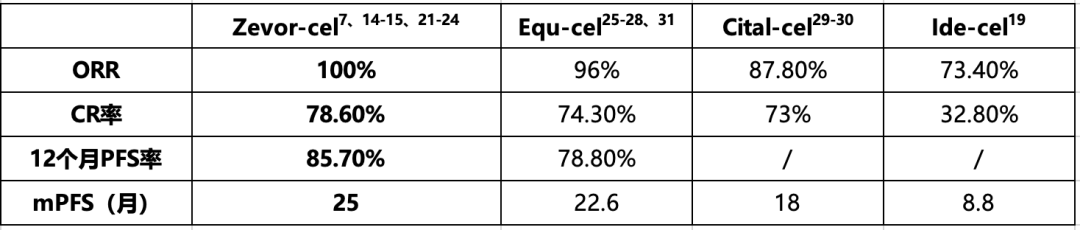
Zevorcabtagene Autoleucel treatment data
Note: ORR: Overall Response Rate; CR: Complete Response; mPFS: median Progression-Free Survival. Zevor-cel: Zevorcabtagene Autoleucel, CT053; Equ-cel: Equecabtagene Autoleucel, CT103A; Cital-cel: Ciltacabtagene Autoleucel, LCAR-B38M; Ide-cel: Idecabtagene Vicleucel, bb2121.
Zevorcabtagene Autoleucel is the only product with a fixed dose, exhibiting a low incidence of Grade 3 or higher CRS, low treatment-related mortality, and favorable safety and tolerability.
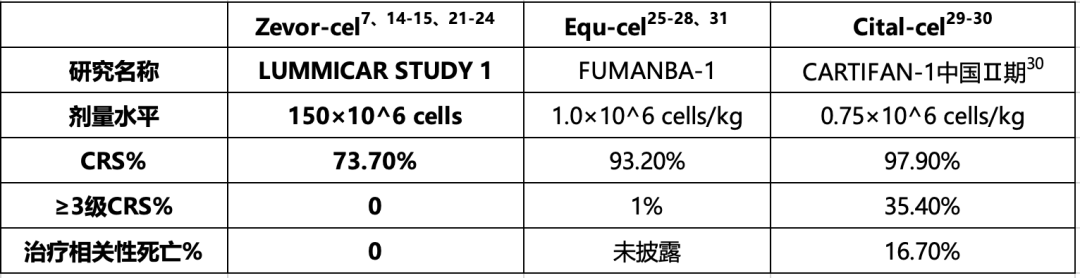
Zevorcabtagene Autoleucel treatment data
Note: CRS: Cytokine Release Syndrome. Zevor-cel: Zevorcabtagene Autoleucel, CT053; Equ-cel: Equecabtagene Autoleucel, CT103A; Cital-cel: Ciltacabtagene Autoleucel, LCAR-B38M; Ide-cel: Idecabtagene Vicleucel, bb2121.
A summary of the clinical trial data for Zevorcabtagene Autoleucel and similar CAR-T products shows:
1) Zevorcabtagene Autoleucel CAR-T therapy can achieve long-term disease control, with a 12-month PFS rate of 85.7%, higher than 78.8% for CT103A. The median PFS reaches 25 months, exceeding 18 months for LCAR-B38M and 8.8 months for bb2121. Zevorcabtagene Autoleucel demonstrates outstanding efficacy, with a 3-year OS rate of 92.9%7, reportedly the highest among similar products.
2) Zevorcabtagene Autoleucel is the only CAR-T product that uses a fixed dose of 150 × 106 CAR BCMA-positive cells, which is more convenient to administer. Additionally, it has a low incidence of Grade 3 or higher CRS and low treatment-related mortality, indicating favorable safety and tolerability.
In this case, the patient was a high-risk MM patient with early relapse after monoclonal antibody therapy, associated with a poor prognosis. After receiving Zevorcabtagene Autoleucel CAR-T cell therapy, the patient achieved a stringent complete response, and long-term follow-up showed a PFS exceeding 60 months. These results demonstrate that Zevorcabtagene Autoleucel is highly effective and safe for treating high-risk MM patients, truly helping to achieve long-term disease control or even cure in the clinical setting and for patients.
