CAR-T Treatment For Multiple Myeloma Has Been Repeatedly Exposed To Potential Carcinogenesis And high Early Mortality Rates, What Is The Prospect Df CAR-T Therapy?
CAR-T Treatment For Multiple Myeloma Has Been Repeatedly Exposed To Potential Carcinogenesis And high Early Mortality Rates, What Is The Prospect Df CAR-T Therapy?
Last week, the FDA released briefing documents from the Oncologic Drugs Advisory Committee (ODAC) meeting regarding Johnson & Johnson’s Carvykti (Ciltacabtagene autoleucel) and Bristol-Myers Squibb’s Abecma (Idecabtagene vicleucel), two CAR-T therapies. The documents highlighted the FDA’s concern over the higher early mortality rates observed in the treatment arms compared to the control arms in the clinical trials for these two CAR-T therapies.
This is not the first time safety concerns have been raised regarding CAR-T therapies. Let’s take a closer look at whether CAR-T therapies can continue to be used.
Higher Early Mortality in CAR-T Treatment Arms
The ODAC meeting focused primarily on two CAR-T therapies: Johnson & Johnson’s Carvykti (Ciltacabtagene autoleucel) and Bristol-Myers Squibb’s Abecma (Idecabtagene vicleucel).
Carvykti
Carvykti is a second-generation CAR-T therapy targeting BCMA. Based on the published CARTITUDE-1 trial (Phase 1b/2 study) results, the FDA approved this therapy in 2022 for the treatment of relapsed/refractory multiple myeloma (r/r MM).

Carvykti
The FDA’s primary concern was the overall survival (OS) data and safety data from Johnson & Johnson’s CARTITUDE-4 trial. As shown in the figure below, released by the FDA, the OS rate in the Carvykti arm was even lower than the standard-of-care arm between 0-11 months. It was only after approximately 14 months that the OS rate in the Carvykti arm surpassed the control arm. Based on these findings, the experts concluded that the assumption of continuous treatment effect for Carvykti was not valid, and therefore, using the hazard ratio (HR) to assess the efficacy of Carvykti in this study was unreliable.
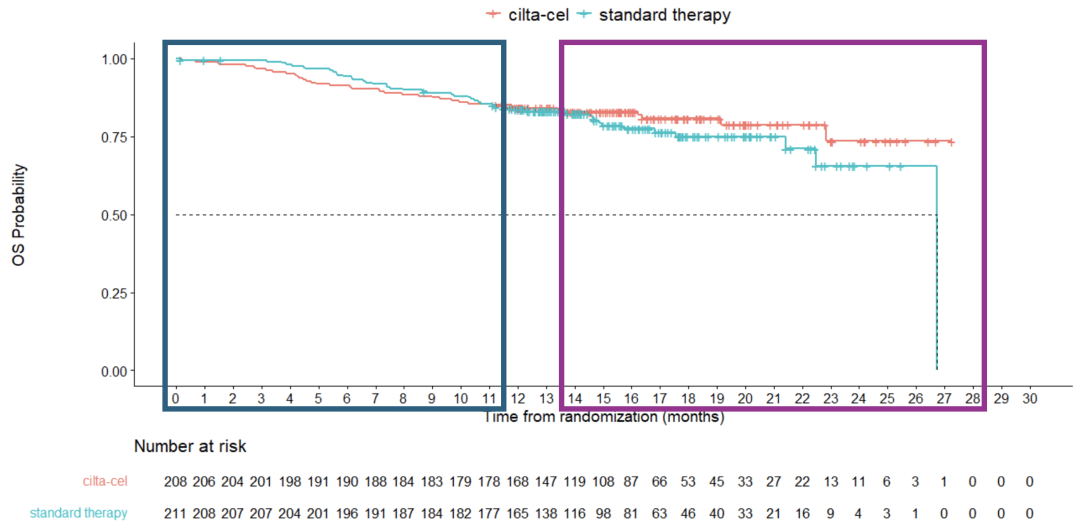
Carvykti treatment data
OS data of cartitude-4 trial (source: FDA briefing document bla 125746/supply 74).
The safety data from the CARTITUDE-4 trial, specifically adverse events (AEs) leading to death, were also a major concern for the FDA. As of November 1, 2022, the rate of AEs leading to death was higher in the Carvykti (Cilta-cel) arm compared to the standard-of-care arm (11% vs. 8%). A more detailed analysis revealed that the increased risk of death in the Carvykti (Cilta-cel) arm persisted for at least 5 months (Piecewise HR: 2.50; 95% CI: 0.99-5.85) and potentially up to 11 months (Piecewise HR: 1.04; 95% CI: 0.62-1.73).
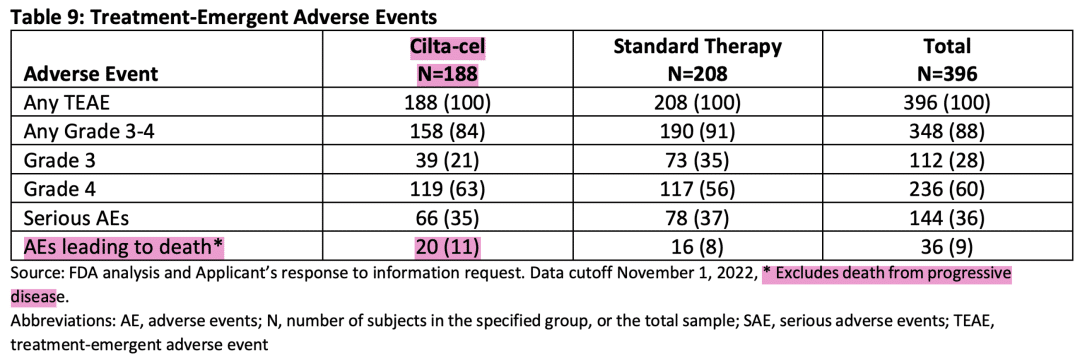
Carvykti adverse events
Adverse event (teae) data during treatment in the cartitude-4 trial (source: FDA briefing document bla 125746/supply 74).
Overall, while Carvykti demonstrated statistically significant progression-free survival (PFS) in the CARTITUDE-4 trial, it remains unclear whether the benefits outweigh the risks, particularly whether additional data is needed for a comprehensive benefit-risk assessment.
Abecma
Abecma is another second-generation CAR-T therapy targeting BCMA, approved by the FDA for the treatment of multiple myeloma in 2021, one year before Carvykti.

Abecma
Similar to the FDA’s concerns regarding Carvykti, the overall survival data from the Phase 3 KarMMa-3 trial for Abecma showed a pattern comparable to the CARTITUDE-4 trial. In the early stages, the OS rate in the Abecma (ide-cell) arm was even lower than the standard-of-care arm, and it was not until 15-18 months that the OS rate in the treatment arm truly surpassed the control arm. Consequently, the assumption of continuous treatment effect for Abecma was also deemed invalid, rendering the use of HR to assess Abecma’s efficacy in the KarMMa-3 trial unreliable.
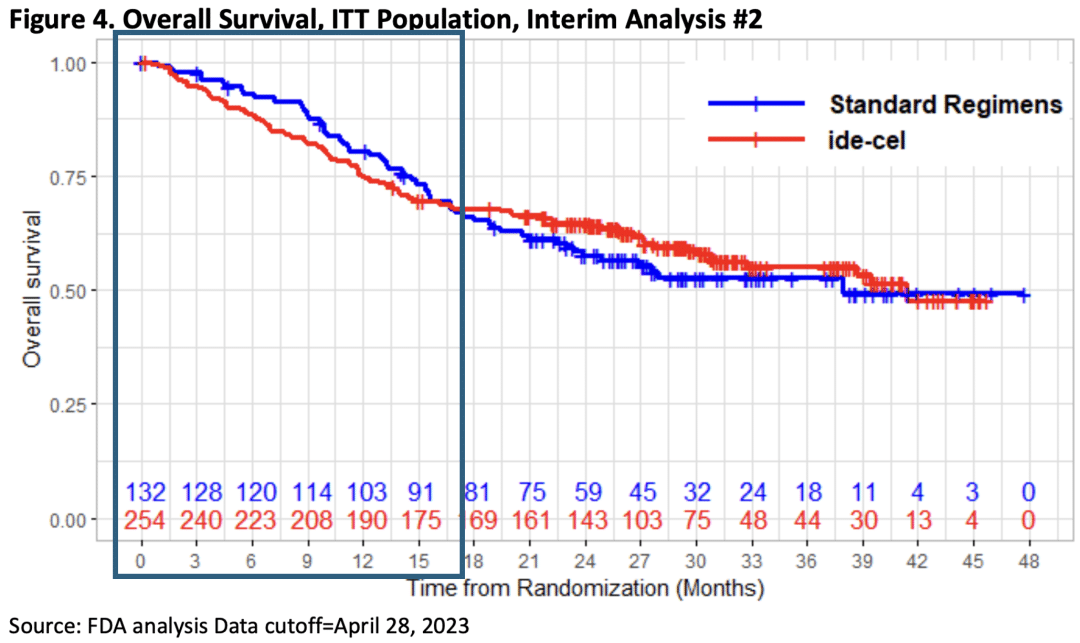
Abecma treatment data
OS data of karmma-3 trial (source: FDA briefing document bla 125736/supply 218).
Similar to the CARTITUDE-4 trial, the KarMMa-3 trial demonstrated statistically significant PFS, but the Abecma (ide-cell) arm had a higher early mortality risk. The rate of AEs leading to death within 90 days of treatment was 2.3% in the Abecma (ide-cell) arm compared to 1.6% in the standard-of-care arm. Additionally, the increased risk of death in the Abecma (ide-cell) arm persisted for at least 9 months (Piecewise HR: 1.65; 95% CI: 0.92-2.97).
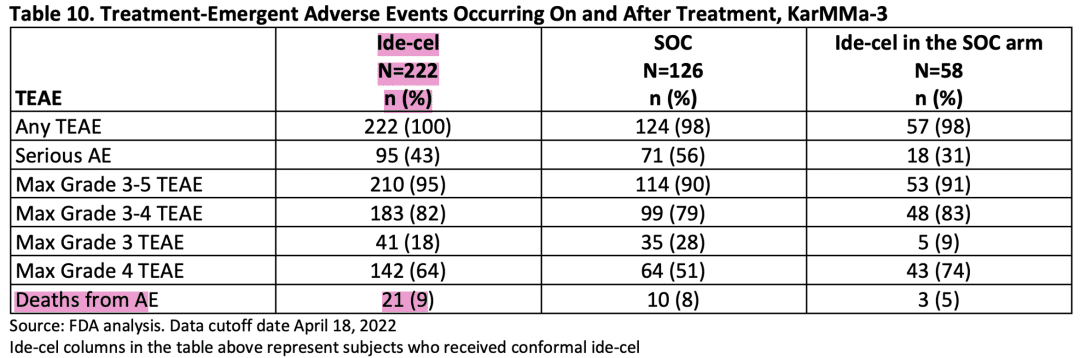
Abecma adverse event
Adverse event (teae) data during treatment in the karmma-3 trial (source: FDA briefing document bla 125736/supply 218).
During last Friday’s ODAC meeting, experts discussed whether the existing data from the KarMMa-3 trial was sufficient to support the benefit-risk profile of Abecma for the proposed indication, particularly whether the early mortality risk associated with Abecma was acceptable in the context of clinical benefit.
14 Patients Developed Cancer Within 2 Years of CAR-T Treatment
On November 28, 2023, the FDA announced that reports from clinical trials and post-marketing adverse event data indicated that some patients developed T-cell malignancies after receiving CAR-T therapies (including those targeting BCMA and CD19).
14 patients who received CAR-T treatment and developed cancer within 2 years
FDA investigated the news that car-t led to the increased risk of T cell malignancies (source: FDA official website).
In an article published in the New England Journal of Medicine in February 2023, staff from the FDA’s Center for Biologics Evaluation and Research (CBER) provided detailed information on this issue. As of December 31, 2023, the FDA had received 22 reports of patients developing T-cell malignancies after receiving CAR-T therapy. These T-cell malignancies included T-cell lymphoproliferative disorders, peripheral T-cell lymphomas, and cutaneous T-cell lymphomas. In the 14 cases with necessary data reported, all cancers occurred within 2 years (1-19 months) of CAR-T infusion, with nearly half occurring within 1 year. In 3 cases where tumor samples were sequenced, the CAR gene was detected.
In late February, researchers from the University of Pennsylvania also reported 16 cases of secondary primary malignancies (SPMs) in patients who had received CAR-T therapy. In one case, a patient with non-Hodgkin lymphoma developed T-cell lymphoma 3 months after receiving anti-CD19 CAR-T therapy. Overall, the 5-year cumulative incidence of solid tumor SPMs was 15.2%, and the 5-year cumulative incidence of hematological SPMs was 2.3% in patients who received commercial CAR-T therapies (these data only indicate an association, not necessarily a causal relationship).
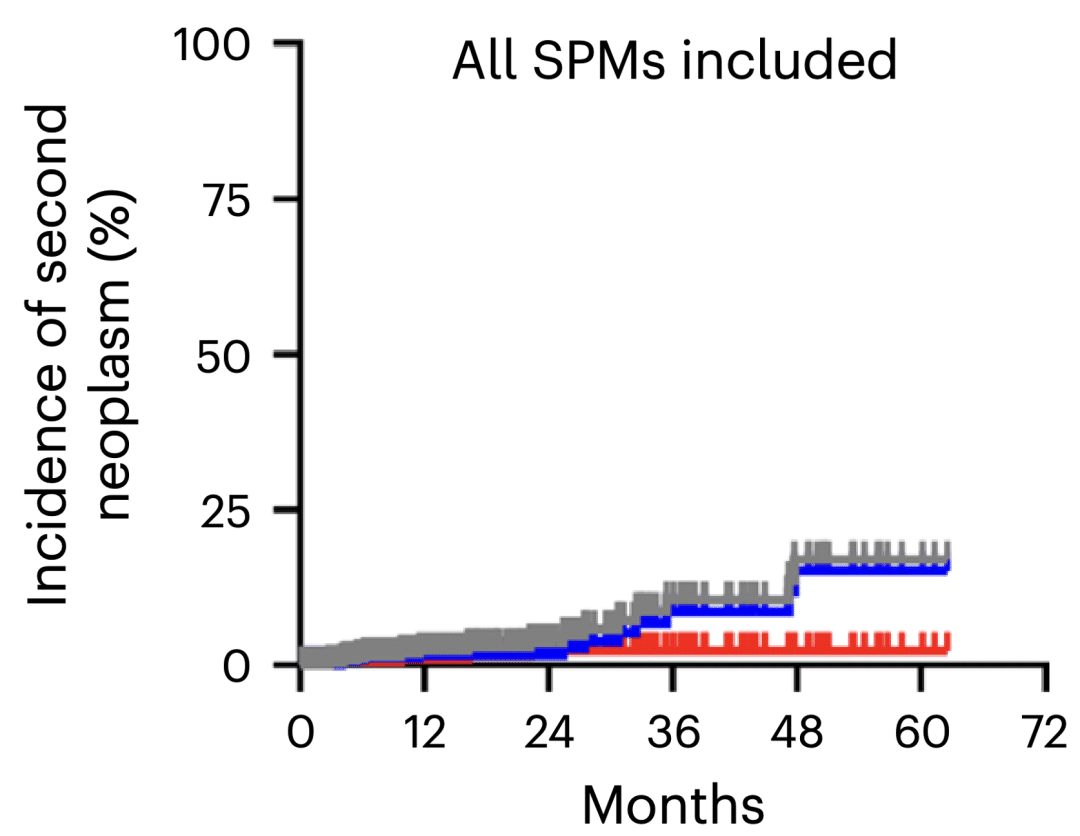
Probability of second primary tumor in patients treated with CAR-T
The cumulative incidence of second primary tumors (SPMS) in patients treated with car-t (source: Nat med. published online January 24, 2024 Doi: 10.1038/s41591-024-02826-w).
Combined Sales of 6 Approved CAR-T Products Exceeded $3.5 Billion
Currently, six CAR-T therapies from companies like Novartis, Gilead (Kite Pharma), Bristol-Myers Squibb, and Johnson & Johnson have been approved by the FDA for the treatment of acute lymphoblastic leukemia.
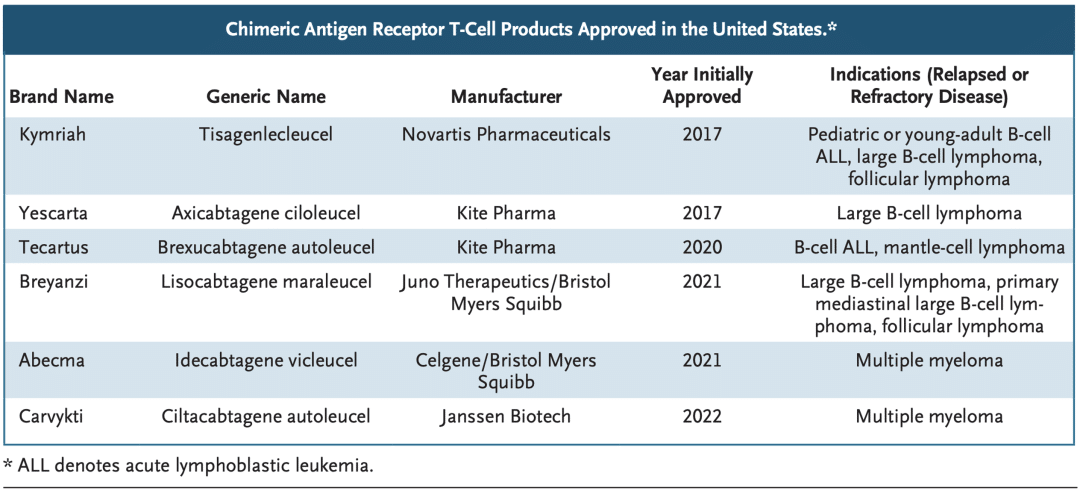
Approved CAR-T products and their indications in the United States
In 2023, Novartis’ Kymriah achieved sales of $500 million, a 5% decrease compared to the previous year, marking the second consecutive year of declining sales for Kymriah.
Gilead’s CAR-T therapies generated over $1.8 billion in sales in 2023. Yescarta saw a 19% increase, reaching $1.5 billion, while Tecartus grew by 24%, reaching $370 million. This significant growth was driven by the high demand from patients with relapsed/refractory large B-cell lymphoma (R/R LBCL), relapsed/refractory acute lymphoblastic leukemia (R/R ALL), and relapsed/refractory mantle cell lymphoma (R/R MCL).
Bristol-Myers Squibb’s Breyanzi generated $364 million in revenue last year, while Abecma saw a 21% increase, reaching $472 million.
Johnson & Johnson’s Carvykti also achieved $500 million in sales last year.
It is estimated that these six approved CAR-T therapies have been infused more than 27,000 times across the United States.
Positive Attitudes Toward CAR-T Therapies Remain
During last Friday’s meeting, 11 experts conducted an anonymous vote on Bristol-Myers Squibb’s Abecma, with an 8:3 majority supporting that the benefits of Abecma outweighed the risks when used as a third-, fourth-, or fifth-line treatment for multiple myeloma. Simultaneously, the experts unanimously agreed that the higher early mortality rate in the Carvykti arm was primarily due to patients dying while waiting for treatment, and therefore, they voted unanimously in favor of Johnson & Johnson’s Carvykti for the treatment of multiple myeloma patients who have received 1-3 prior lines of therapy.
In theirFDA officials stated in the New England Journal of Medicine article that even if all 22 cases of secondary malignancies were related to CAR-T therapy, given that over 27,000 CAR-T infusions have been administered, the risk of developing T-cell malignancies after CAR-T treatment remains low.
As the duration of potential CAR-T-related adverse effects is still unclear, the FDA officials recommended lifelong monitoring for malignancies in all patients receiving CAR-T therapies or participating in clinical trials.
Despite these concerns, professional organizations, researchers, and clinical experts remain supportive of CAR-T therapies. Organizations such as the American Society for Transplantation and Cellular Therapy (ASTCT), European Society for Blood and Marrow Transplantation (EBMT), International Society of Cell Therapy (ISCT), Center for International Blood and Marrow Transplant Research (CIBMTR), and current and former chairs of the Parker Institute for Cancer Immunotherapy (PICI), along with immunotherapy researchers and clinicians, believe that for most patients, the benefits of CAR-T therapies outweigh the risks.
In summary, as a last hope for patients with hematological malignancies, even with a potential increased risk of developing cancer, the benefits of CAR-T therapies are generally considered to outweigh the risks.
Content comes:读懂医价值
