A Brief Analysis of Carvykti Multiple Myeloma
A Brief Analysis of Carvykti Multiple Myeloma
Legend Biotech was founded in 2014 and is a multinational biopharmaceutical company focused on the research, development, and production of cell immunotherapies for cancer and other indications. The company went public on the NASDAQ (LEGN.O) in 2020.
Leveraging the team’s extensive research and development experience, the company continuously explores the potential of cell therapy to treat diseases that currently lack effective treatments, such as hematological malignancies, solid tumors, infectious diseases, and autoimmune diseases. The company has a relatively rich pipeline, and its current marketed product is Carvykti.
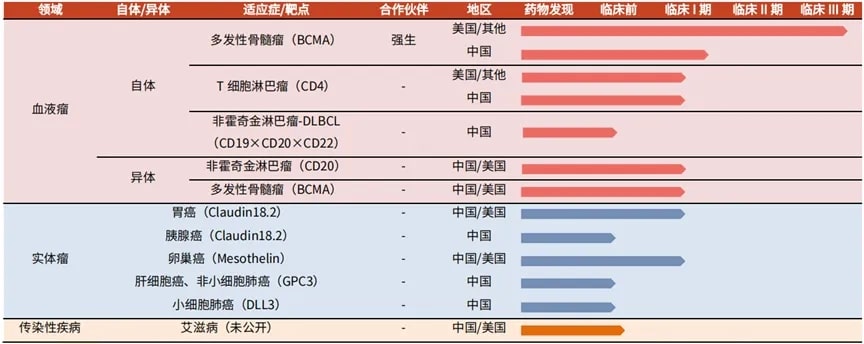
Figure 1 Legend Biotech’s research and development pipeline
BCMA is a specific target for multiple myeloma, and therapies targeting BCMA are considered an effective way to improve the quality of life for patients with multiple myeloma. In the first half of 2022, Legend Biotech’s BCMA CAR-T product Carvykti, co-developed with Johnson & Johnson, received marketing approvals from the FDA and EMA, respectively, for the treatment of 5L+ and 4L+ relapsed/refractory multiple myeloma based on its outstanding clinical trial data. Carvykti is currently considered the best-in-class product.
Carvykti adopts a second-generation CAR-T structure with a murine-derived scFv and incorporates the 4-1BB co-stimulatory domain. It uses a lentiviral vector and is designed with two single-domain antibodies targeting BCMA extracellularly, resulting in stronger affinity. Carvykti’s FDA approval was primarily based on data from the CARTITUDE-1 clinical trial, a Phase Ib/II study conducted in the United States and Japan. Legend Biotech’s registrational Phase II CARTIFAN-1 trial in China is also ongoing.
The CARTITUDE-1 study enrolled 97 patients who had received a median of six prior lines of therapy, including 29 patients in Phase Ib and 68 patients in Phase II. At the ASCO 2022 meeting, Legend Biotech updated the CARTITUDE-1 clinical trial data, showing an ORR of 98% with a median follow-up of 28 months. Earlier follow-up data showed that 83% of patients achieved sCR at the median follow-up of 28 months, with a 2-year PFS and OS rate of 55% and 70%, respectively. Furthermore, among 61 patients evaluable for MRD, 92% achieved MRD negativity, with 68% sustaining MRD negativity for over 6 months and 55% sustaining it for over 12 months. In terms of safety, 5.1% experienced Grade ≥3 CRS, and 12.3% experienced neurotoxicity, demonstrating a favorable safety profile.
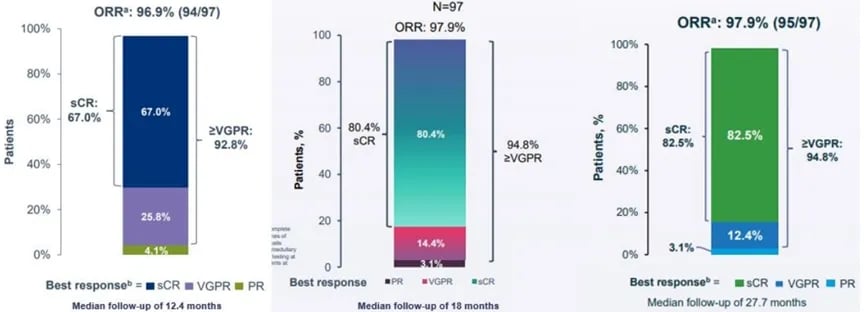
Figure 2 Clinical remission deepened over time in the CARTITUDE-1 study
Additionally, Legend Biotech is conducting a Phase II trial (CARTIFAN-1) in China, which was initially registered on clinicaltrials.gov in November 2018. The study plans to enroll 130 patients and is estimated to be completed by November 2022. Earlier, Legend Biotech conducted the Phase I LEGEND-2 study in China, and the four-year follow-up results were recently published in the Journal of Hematology & Oncology.
The LEGEND-2 study enrolled 74 patients with relapsed/refractory multiple myeloma. The four-year follow-up results showed an ORR of 87.8%, a CR rate of 73%, a mPFS of 18 months, a mDOR of 23.3 months, a 24-month OS rate of 63.4%, and an MRD negativity rate of 67.6%. In terms of safety, 91.9% of patients experienced CRS, with a Grade ≥3 CRS rate of 9.5%. One patient experienced Grade 1 central nervous system adverse events, and no Grade ≥3 neurotoxicity occurred.
