Current Status and Future Trends of CAR-T For Myeloma
Current Status and Future Trends of CAR-T For Myeloma
On March 2-3, 2024, the 7th Beijing Thrombosis and Hemostasis Conference and the 5th Beijing Hematologic Malignancies and Immunology Summit, hosted by the National Clinical Research Center for Blood Diseases, Institute of Hematology & Blood Diseases Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, was successfully held in Beijing. Numerous domestic and international hematology experts and scholars shared the latest advances in the treatment of leukemia, coagulation disorders, and malignant hematological diseases through thematic reports, paper exchanges, case presentations, and expert panels. At the conference, Professor Fu Qiqu from the First Affiliated Hospital of Soochow University gave an insightful presentation on “Current Status and Future Trends of CAR-T Therapy for Multiple Myeloma.” The following is a summary of her presentation, compiled by “Tumor Outlook – Hematology News” for readers’ reference.
Current Status of BCMA CAR-T in Later Line Treatment for MM
Chimeric antigen receptor T-cell (CAR-T) therapy has become a transformative treatment for hematological malignancies, marking a milestone event. B-cell maturation antigen (BCMA) is theoretically expressed in all newly diagnosed (NDMM) or relapsed/refractory multiple myeloma (RRMM) patients, making it a key target for MM treatment.
Compared to acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma (NHL), CAR-T research in the MM field was relatively late. In March 2021, the US FDA approved BMS’s Abecma (idecabtagene vicleucel, Ide-cel) for the treatment of adult RRMM patients who had previously received at least four lines of therapy, marking the first FDA-approved CAR-T therapy for multiple myeloma. Subsequently, in 2022, the FDA approved JnJ/Legend Biotech’s CARVYKTI (Ciltacabtagene Autoleucel, Cilta-cel) for the treatment of RRMM, with the same indication as Ide-cel. Following this, in 2023-2024, two BCMA CAR-T therapies, Equecabtagene Autoleucel and Zevorcabtagene Autoleucel, were approved in China for adult RRMM patients who have progressed after at least three lines of treatment.
So, what are the efficacy and safety profiles of these four approved BCMA CAR-T therapies in later-line RRMM treatment? The KarMMa, CARTITUDE-1, LUMMICAR-1 (Phase I/II), and FUMANBA-1 (Phase I/II) studies have provided relatively clear and objective results.
KarMMa Study (idecabtagene vicleucel)
Idecabtagene vicleucel’s approval was based on the favorable results from the KarMMa study, a phase II, single-arm, multicenter clinical trial that enrolled 140 RRMM patients, of whom 128 received ide-cel treatment. The median age was 61 years, 39% had extramedullary disease, 16% were in R-ISS stage III, and 35% had high-risk cytogenetics.
With a median follow-up of 13.3 months, the results showed:
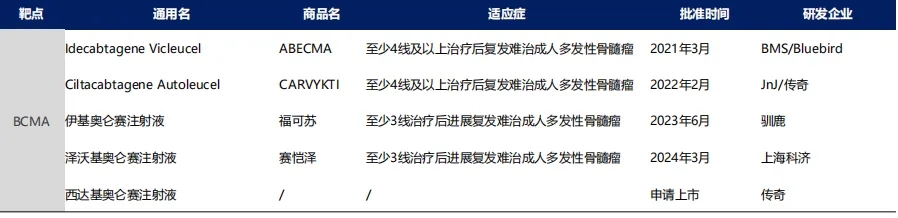
① According to the IRC assessment, 94 patients achieved a response after receiving ide-cel treatment, with an objective response rate (ORR) of 73% (95% CI: 66-81%; P<0.001). Of these, 42 (33%) achieved complete response/stringent complete response (CR/sCR), and 67 (52%) achieved at least a very good partial response (VGPR). Of the 42 patients who achieved CR/sCR, 33 (79%) achieved minimal residual disease (MRD) negativity at the 10^-5 level.
② The overall median progression-free survival (mPFS) was 8.8 months, with the 450×10^6 dose group achieving an mPFS of 12.1 months.
③ The adverse events (AEs) associated with ide-cel treatment mainly included neutropenia (91%), anemia (70%), and thrombocytopenia (63%). Additionally, 84% experienced cytokine release syndrome (CRS), with 5% being grade 3 or higher, and 18% experienced neurotoxicity, with 3% being grade 3, but none higher than grade 3.

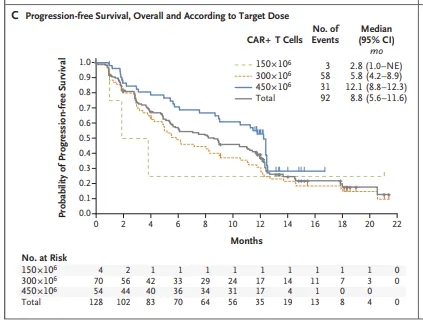
N Engl J Med. 2021 Feb 25;384(8):705-716.
CARTITUDE-1 Study (Ciltacabtagene Autoleucel)
At the 2023 EHA meeting, Professor Nikhil Munshi et al. updated the final follow-up results of the CARTITUDE-1 study, a single-arm, multicenter, phase Ib/II clinical trial that enrolled 97 RRMM patients who had previously received at least three lines of therapy and were treated with Cilta-cel infusion. The median age was 61 years, the median number of prior lines was 6, the extramedullary plasmacytoma rate was 19.6%, 25% had high-risk cytogenetics, and 88% were triple-refractory. With a median follow-up of 33.4 months, the results showed: ① The best overall response rate (ORR) assessed by the IRC was 97.9%, with a CR or better rate of 82.5% and a VGPR or better rate of 94.9%. The median duration of response (DOR) was 33.9 months, the median progression-free survival (PFS) was 34.9 months, and the median overall survival (OS) has not been reached.
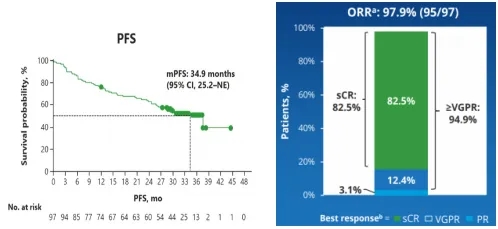
Munshi, et al. 2023 EHA, Oral :CARTITUDE-1 final result: Phase Ib/2 study of citacabtagene autoleucel in heavily pretreated patients with relapsed/refractory multiple myeloma.
LUMMICAR-1 (Zevorcabtagene Autoleucel)
LUMMICAR-1 Phase I: 14 RRMM patients (previously treated with at least one IMiD and PI, and progressed after the last treatment) were enrolled and received Zevorcabtagene Autoleucel cell infusion. The median age was 54 years, the median number of prior treatments was 6, 50% had high-risk cytogenetics, 14.3% had extramedullary disease, and 14.3% were in ISS stage III. With a median follow-up of 37.7 months, the results showed: ORR was 100%, sCR rate was 71.4%, and ≥CR rate was 78.6%; all patients who achieved ≥CR were MRD-negative. The overall median PFS was 25 months, and the median PFS for sCR/CR patients was 26.9 months; the median DOR was 24.1 months; the median OS was not reached.
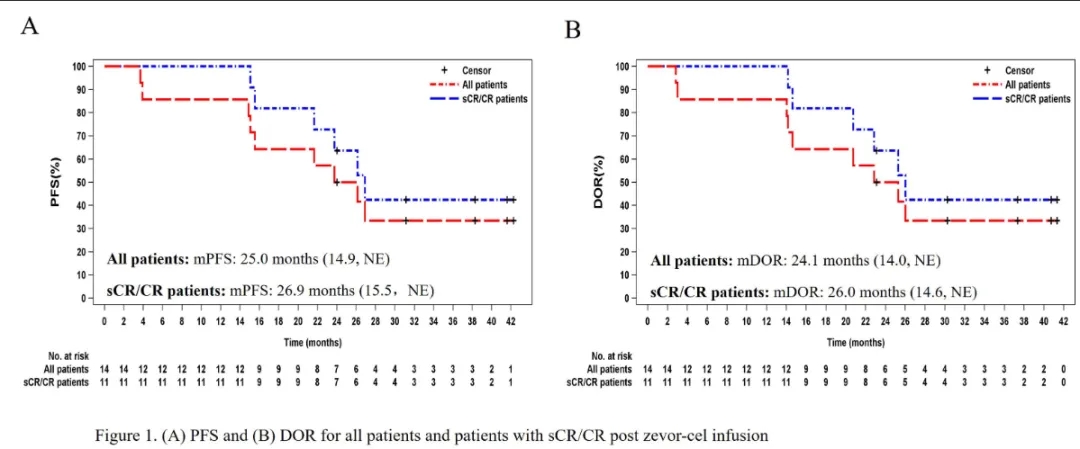
Chengcheng Fu, et al. 2023 ASH, Poster 4845
LUMMICAR-1 Phase II: 102 RRMM patients who had previously received ≥3 lines (including at least one IMiD and PI, and progressed after the last treatment) were enrolled and received 1.5×10^8 Zevorcabtagene Autoleucel cell infusion. The median age was 59.5 years, the median number of prior lines was 4, 38.2% were in ISS stage III, 45.1% had high-risk cytogenetics, 10.8% had extramedullary disease, 89.2% were double-refractory, and 22.5% were triple-refractory.
With a median follow-up of 12.6 months, the results showed:
① The IRC-assessed ORR was 91.7%; the CR/sCR rate was 56.7%; the ≥VGPR rate was 88.3%; the median PFS and DOR have not been reached.
② Of the 51 patients who achieved VGPR or better, 92.2% achieved MRD negativity, and all 34 patients who achieved CR or better were MRD-negative (10^-5).
FUMANBA-1 Phase I/II (Equecabtagene Autoleucel)
In the FUMANBA-1 study, 103 RRMM patients received Equecabtagene Autoleucel cell infusion. The median age was 58 years, the median number of prior lines of treatment was 4, 18.4% had previously received CD38 monoclonal antibody therapy, 48.5% had high-risk cytogenetics, and 9.7% had extramedullary disease. In the phase II trial, with a median follow-up of 8.7 months for 62 patients, the IRC-assessed results showed: the 6-month best overall response rate was 90.3%, the complete response (CR) rate was 58.1%, and the rate of very good partial response (VGPR) or better was 87.1%; the median progression-free survival (PFS) and median duration of response (DOR) were not reached, and the median overall survival (OS) was not reached. 57 patients (91.9%) achieved minimal residual disease (MRD) negativity, and all 34 patients who achieved CR or better were MRD-negative (10^-5).
BCMA CAR-T in Early Line Treatment of Multiple Myeloma
While CAR-T therapy has shown promising efficacy in RRMM, relapse remains common, particularly in rapidly progressing or late-stage MM patients. Available data suggest that RRMM patients who progress after prior BCMA-targeted therapy have poor outcomes with subsequent CAR-T treatment, making the sequencing of CAR-T and other treatment regimens challenging.
The early use of BCMA CAR-T in MM patients is currently a hot research topic. Early application of CAR-T when T cells are potentially healthier and MM is less aggressive could be advantageous. Currently, the main clinical trials investigating early use of BCMA CAR-T are the KarMMa-3 study (2-4 prior lines) and the CARTITUDE-4 study (1-3 prior lines).
KarMMa-3 Study (Early Use of Idecabtagene Vicleucel)
KarMMa-3 is a phase III study that enrolled 386 RRMM patients who had received an average of 2-4 prior lines of therapy (including IMiDs, PIs, and daratumumab). Patients were then randomized 2:1 to receive ide-cel (n=225) or standard treatment (including DPd, DVd, IRd, Kd, and EPd regimens, n=126), with cross-over allowed. Baseline patient characteristics: median age 63 years, median time from initial diagnosis approximately 4 years, approximately 85% had prior autologous stem cell transplant (ASCT), 12% had R-ISS stage III, 24% had extramedullary disease, and approximately 65% had high-risk cytogenetics. Updated follow-up results presented at the 2023 ASH meeting showed:
Efficacy:
① Ide-cel significantly improved median PFS in the ITT population compared to standard treatment (13.8 months vs. 4.4 months, p<0.0001), reducing the risk of disease progression and death by 51%.
② In the standard treatment arm, 56% of patients crossed over to receive ide-cel. Median OS was not reached in either arm, but showed improvement with ide-cel (HR=0.83).
③ In the ITT population: ORR was 71.3% (CR 43.7%) with ide-cel and 42.4% (CR 5.3%) with standard treatment.
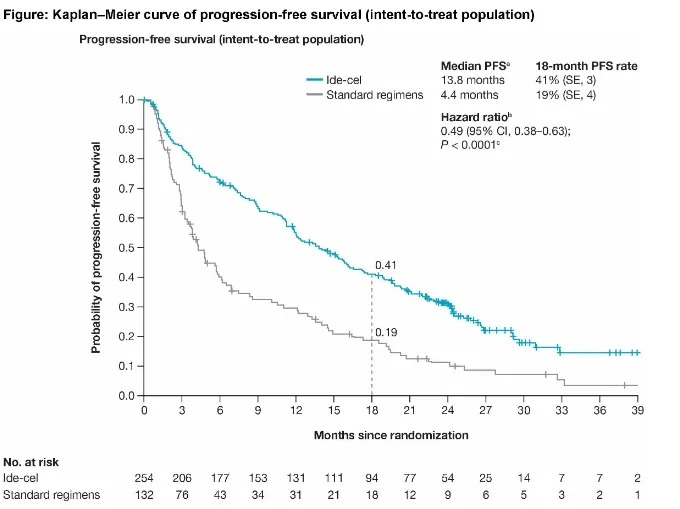
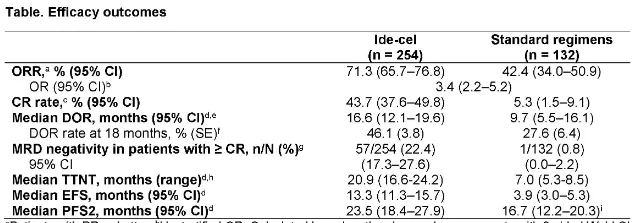
Paula Rodríguez Otero, et al. 2023 ASH, Oral 1028
Safety:
① Rates of severe adverse events (SAEs) were 47% with ide-cel and 41% with standard treatment. Deaths due to disease progression were 25% and 28%, respectively. In the ide-cel arm, any-grade CRS occurred in 88% (grade ≥3 in 4%), neurotoxicity in 15% (grade ≥3 in 3%), and infections in 56% (grade ≥3 in 22%).
② In the ide-cel arm, there were 2 treatment-related secondary malignancy deaths, with no T-cell-related secondary malignancies reported.
CARTITUDE-4 Study (Early Use of Ciltacabtagene Autoleucel)
At the 2023 ASCO and ASH meetings, Binod Dhakal, M Hasib Sidiqi, and colleagues reported follow-up results from the CARTITUDE-4 study, which compared early use of ciltacabtagene autoleucel (cita-cel, 1-3 prior lines) vs. standard treatment (PVd or DPd) in lenalidomide-refractory MM patients. At ASCO, with a median follow-up of 15.9 months, the results showed:
Efficacy:
① Cita-cel significantly improved median PFS in the ITT population compared to standard treatment (not reached vs. 11.8 months, P<0.0001), reducing the risk of disease progression and death by 74%.
② In the ITT population: ORR was 84.6% (CR 73.1%) with cita-cel and 67.3% (CR 21.8%) with standard treatment. The MRD negativity rate in the ITT population was 60.6% with cita-cel and 15.6% with standard treatment.
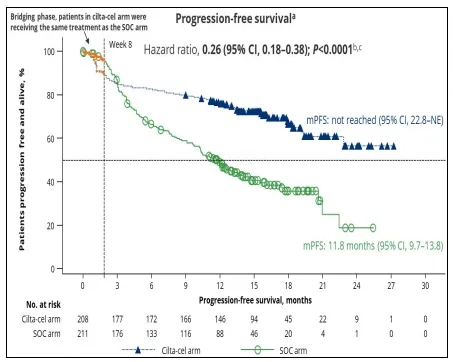
Binod Dhakal,et al. 2023 ASCO, Oral :Phase 3 results from CARTITUDE-4:cilta-cel versus standard of care(PVd or DPd)in lenalidomide-refractory multiple myeloma.
Safety:
① Hematologic toxicities were the most common adverse events; secondary malignancies are also a long-term concern. The most common CAR-T-related toxicities were CRS (any grade 76.1%, grade 3 1.1%), CAR-T neurotoxicity (20.5%, grade 3/4 2.8%), and ICANS (4.5%, no grade 3/4).
② Other neurotoxicities occurred in 17% of patients (grade 3/4 2%); including cranial nerve palsies 9.1% (grade 3/4 1.1%), peripheral neuropathy 2.8% (grade 3/4 0.6%), and one case of grade 1 MNT.
The ASH presentation showed similar efficacy and safety results for early cita-cel vs. standard treatment.
Current Status of GPRC5D CAR-T Development
GPRC5D is an orphan receptor belonging to the GPCRs family. In normal tissues, GPRC5D is expressed only in the immune-privileged hair follicle area, but it is highly expressed on the surface of multiple myeloma (MM) cells. In recent years, GPRC5D-targeted CAR-T has become a hot topic in MM treatment. However, it’s important to note that BCMA has already been established as a target for MM, and several BCMA CAR-T products have been approved by the FDA. Therefore, it’s crucial to validate that GPRC5D expression is independent of BCMA.
To address this issue, the Memorial Sloan Kettering Cancer Center used immunohistochemistry to analyze bone marrow samples from 83 MM patients. By comparing the expression ratio of GPRC5D and BCMA in each sample, they concluded that GPRC5D expression is independent of BCMA expression.
CC-95266-MM-001 Study (BMS-986393)
The CC-95266-MM-01 study is the first-in-human phase I clinical trial of GPRC5D-targeted CAR-T, enrolling 84 RRMM patients who had received 3 prior treatment regimens and progressed within 12 months of their last treatment. The dose-escalation phase enrolled 36 patients, and the dose-expansion phase enrolled 48 patients. The recommended phase II dose was 150×10^6 CAR-T cells, with 26 patients enrolled in this cohort. The median age was 63 years, the median number of prior lines of therapy was 5, 40.5% had high-risk cytogenetics, 44% had extramedullary disease, 73.8% were triple-refractory, 32.6% were penta-refractory, and 20.2% were refractory to BCMA-targeted therapy.
The results showed:
① In the 73 patients who received the infusion across all dose levels, the ORR was 88% (CR: 45%). In the RP2D cohort (150×10^6 CAR-T cells), the ORR was 91% (CR: 48%).
② In the 23 patients in the RP2D cohort, the response rate was 87% for the 15 patients who had not previously received BCMA-targeted therapy, and 100% for the 8 patients who had received BCMA-targeted therapy. With a median follow-up of 9 months, the median DOR was 13 months, and 67% of responders remained in response.
③ Grade ≥3 neutropenia occurred in 61.9%, anemia in 29.8%, and thrombocytopenia in 26.2%; the median time to recovery from grade ≥3 cytopenias was 46 days. Any-grade CRS occurred in 76.2%, with 3.6% grade ≥3; the median time to CRS onset was day 3, and the median duration was 4 days. No grade ≥3 CRS or HLH occurred in the RP2D cohort. Grade ≥3 ICANS occurred in 2.4%, with no grade ≥3 ICANS in the RP2D cohort.
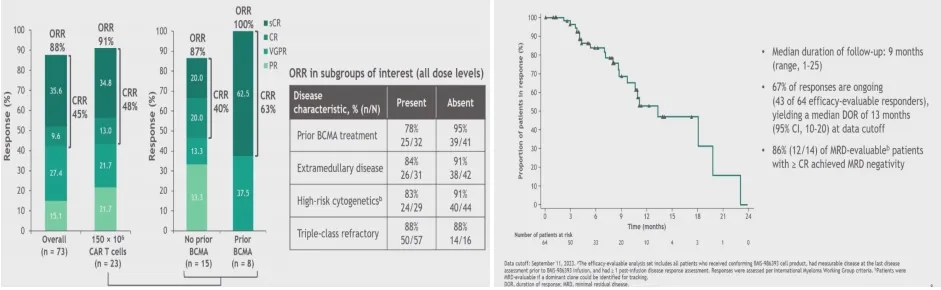
Susan Bai,et al.2023 ASH, Oral 219
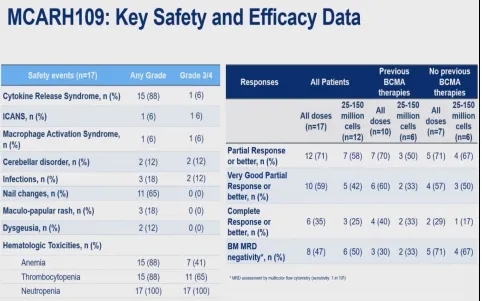
MCARH109 Study
MCARH109 is a phase I clinical trial of GPRC5D-targeted CAR-T, enrolling 17 RRMM patients who had received 3 classes of drugs and progressed after 3 lines of therapy. Patients were assigned to dose levels of 25×10^6, 50×10^6, 150×10^6, and 450×10^6 CAR-T cells. Among the 17 patients, the median age was 60 years, the median number of prior lines was 6, 77% had high-risk cytogenetics, 41% had extramedullary disease, 94% were triple-refractory, 59% had previously received BCMA-targeted therapy, and 47% had previously received CAR-T therapy. CRS occurred in 88% (6% grade ≥3), ICANS in 6% (1 patient), grade ≥3 infections in 12%, nail changes in 65% (no grade ≥3); grade ≥3 neutropenia occurred in 100%, and grade ≥3 thrombocytopenia in 65%.
2023 ASH, Oral 336. Xiaoli Mi et al.Genetic Basis of Relapse after GPRC5D-Targeted CAR T Cells
Future Trends in MM CAR-T Therapy
The future trends in MM CAR-T therapy can be summarized as follows:
01. Multi-target CAR-T: In the MM field, while BCMA CAR-T is relatively mature, as research progresses, CAR-T targeting other new targets will emerge, such as dual-target CAR-T targeting BCMA and CD19, and modular CAR-T.
02. Universal CAR-T entering clinical research: Allogene’s universal CAR-T product ALLO-715, with TCR and CD52 gene knockouts, achieved an ORR of 71% and CR of 25% in MM treatment. Additionally, Cellectis’ universal CAR-T product UCARTCS1A, with TCR and CS1 knocked out, achieved an ORR of 40% in MM treatment.
03. Development of in vivo CAR-T: In the future, patients receiving treatment may only need to be injected with a virus infecting T cells, integrating the genes required for targeting tumor cells into the T cell genome to modify them, allowing CAR-T cells to be generated in vivo. Preclinical studies have shown that in vivo CAR-T cells can eliminate B cells in monkeys, and human trials will begin.
Content Source:《肿瘤瞭望–血液时讯》编辑部
