The Beacon of BCMA CAR-T Myeloma Therapy
The Beacon of BCMA CAR-T Myeloma Therapy
Multiple Myeloma Awareness Month is a global social awareness initiative held annually in March to raise awareness about multiple myeloma (plasma cell malignancy). Multiple myeloma is the second most common hematological malignancy worldwide and is most prevalent among African Americans. However, many people have never heard of this disease and often confuse it with melanoma (skin cancer). Over the past decade, with the advent of novel therapeutic agents, survival outcomes for multiple myeloma (MM) patients have significantly improved. Nevertheless, MM remains an incurable plasma cell malignancy, and nearly all MM patients inevitably relapse due to drug resistance. Encouragingly, in recent years, B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor T (CAR-T) cell therapy has achieved remarkable success in treating relapsed/refractory (R/R) MM, bringing new hope to R/R MM patients.
What is Multiple Myeloma
Multiple myeloma (MM) is a malignant plasma cell neoplasm characterized by the clonal proliferation of malignant plasma cells in the bone marrow, accompanied by the overproduction of a monoclonal immunoglobulin (M-protein), and associated end-organ damage, accounting for approximately 10% of hematological malignancies. With a deepening understanding of the pathogenesis of MM and the application of novel therapeutic agents, including selective nuclear export inhibitors, survival outcomes for MM patients have greatly improved. However, almost all MM patients eventually relapse. Particularly, patients with relapsed or refractory (R/R) disease, extramedullary disease (EMD), or high-risk cytogenetic abnormalities typically have a poorer prognosis. Moreover, clonal evolution of MM cells frequently occurs under the selective pressure of treatment, which may lead to disease progression and resistance to conventional therapies. Thus, there is an urgent need for novel treatment approaches to address R/R MM.

CAR-T Cell Therapy for MM
In recent years, chimeric antigen receptor T (CAR-T) cell therapy, as a highly promising immunotherapy, has profoundly transformed the treatment landscape of hematological malignancies. To generate CAR-T cells capable of specifically recognizing tumor surface antigens, T cells derived from patients or healthy donors are genetically engineered to express a tumor-targeting receptor called a chimeric antigen receptor (CAR). The CAR structure comprises a single-chain variable fragment (scFv) that can specifically recognize tumor surface antigens without the need for MHC-restricted antigen presentation. Similar to effector T cells, CAR-T cells can mediate tumor cytotoxicity through various mechanisms. Currently, B-cell maturation antigen (BCMA) is the most successful target for CAR-T cell therapy in MM, and anti-BCMA CAR-T cell therapy has achieved unprecedented efficacy in R/R MM patients, bringing new hope to these R/R MM patients.
Instances of Efficacy
To date, two anti-BCMA CAR-T cell products, idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel), have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of R/R MM. In recent years, as more and more CAR-T cell clinical trials have been conducted, CAR-T-related adverse events have gradually been recognized and are generally manageable, such as cytokine release syndrome, CAR-T cell-related encephalopathy syndrome, cytopenias, and infections. However, there are still some substantial challenges, such as resistance to anti-BCMA CAR-T cell therapy and limited accessibility to CAR-T cell therapy. Therefore, many research efforts are ongoing to explore effective strategies.
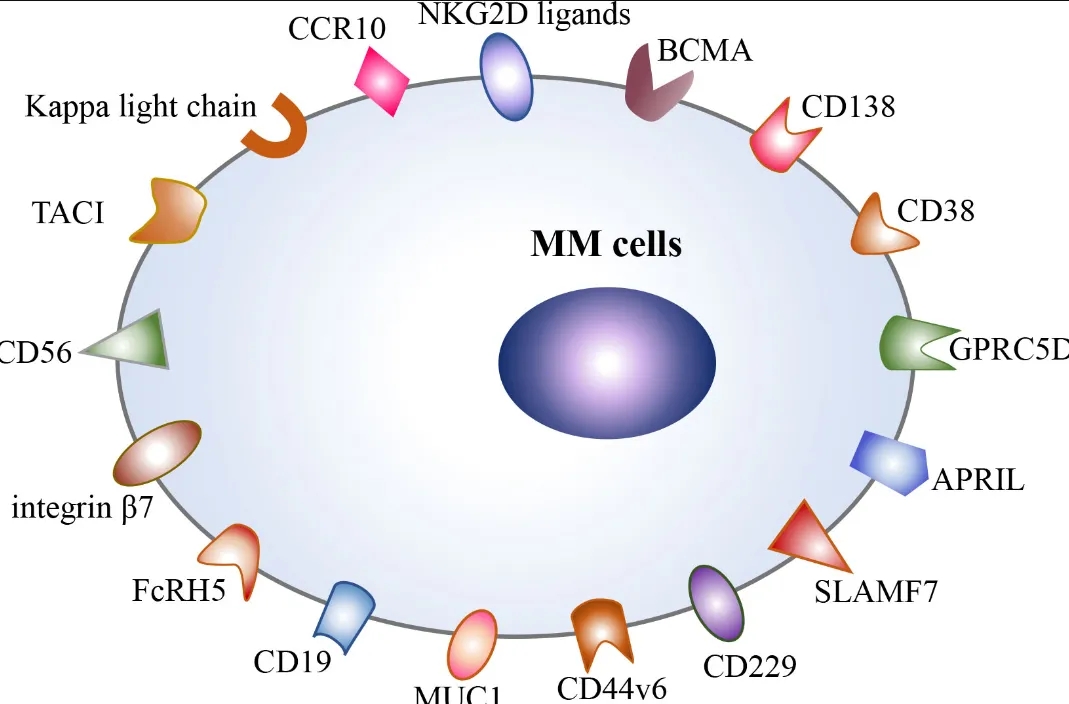
B-cell Maturation Antigen (BCMA) CAR-T Targets
Idecabtagene Vicleucel (Ide-Cel, bb2121)
Idecabtagene vicleucel, also known as Ide-cel and bb2121, is the second-generation FDA-approved BCMA-targeted CAR-T cell therapy. Ide-cel consists of autologous T cells transduced with a lentiviral vector expressing the BCMA CAR, and it was approved in 2021. Notably, in an ex vivo study involving relapsed and refractory MM (RRMM) patients, Ide-cel exhibited rapid and sustained tumor eradication. Based on these promising results, a phase 1 clinical trial (CRB-401, NCT02658929) was conducted in heavily pretreated and refractory RRMM patients who had received three or more prior lines of therapy. The trial results highlighted the efficacy of Ide-cel in this patient population, characterized by an impressive overall response rate (ORR) of 73% and a complete response rate (CR) of 33%.
Ciltacabtagene Autoleucel
Cilta-cel is a gene-engineered BCMA-targeted CAR-T cell therapy. It belongs to the second-generation CAR, featuring a dual-binding BCMA scFv structure with two domains. Cilta-cel is currently indicated for RRMM after idecabtagene vicleucel (bb2121) CAR-T cell therapy. Cilta-cel is the second FDA-approved immunotherapy for late-stage MM patients. The FDA granted approval for Cilta-cel for adult MM patients on February 28, 2022. This decision was based on the results from the CARTITUDE-1 study (NCT03548207), which included phase 1b and phase 2 open-label trials. These trials involved individuals who had received at least three prior therapies. Of the 113 enrolled participants, 97 received a single infusion of cilta-cel at the target dose of 0.75 × 106 CAR-T cells/kg (range 0.5-1.0 × 106). With a median follow-up extended to 12.4 months, the observed 12-month overall survival (OS) and progression-free survival (PFS) were 89% and 77%, respectively. Of the 97 patients, 73 received bridging therapy, primarily various combinations of Bortezomib, Pomalidomide, Carfilzomib, corticosteroids, and Daratumumab. Notably, 49% of patients had increasing tumor burden, and only 45% responded to the bridging therapy. The cumulative results from CARTITUDE-1 and CARTITUDE-2 underscore the potential of cilta-cel as a promising treatment option for RRMM patients.
JCARH125 (Orva-Cel)
Orva-cel is another BCMA CAR-T cell product transduced with a lentiviral vector expressing the BCMA CAR construct. The construct comprises a unique BCMA-specific human single-chain variable fragment, a costimulatory domain (4-1BB), and a CD3ζ signaling domain. A phase 1/2 trial (EVOLVE trial) (NCT03430011) studied 51 RRMM patients who received orva-cel at doses of 300, 450, and 600 × 106 CAR-T cells after lymphodepletion with Cyclophosphamide and Fludarabine. Efficacy data showed an ORR of 91%. However, two patients experienced dose-limiting toxicities: one patient developed a grade 3 neurological event after receiving 300 × 106 CAR-T cells, and another patient experienced grade 4 neutropenia after receiving 300 × 106 CAR-T cells. Additionally, 14% of patients developed grade 3 or higher infections. Nevertheless, cytokine release syndrome (CRS) was manageable.
Johnson & Johnson – 4528(LCAR-B38M)
JNJ-68284528, also known as JNJ-4528, is a second-generation bispecific chimeric antigen receptor (CAR) T-cell therapy aimed at specifically targeting the B-cell maturation antigen (BCMA). It possesses two distinct epitopes and a costimulatory domain structure, enhancing its binding kinetics with BCMA-expressing cells. A single-arm, multicenter, open-label phase 1 and phase 2 study has been initiated in China to evaluate its efficacy and safety in patients with relapsed or refractory multiple myeloma (RRMM). The first-in-human study (NCT03090659) conducted in 74 RRMM patients demonstrated durable responses and manageable safety.
A phase 2 clinical trial named CARTIFAN-1 (NCT03758417) has been launched in China to further explore its therapeutic potential. The safety profile of LCAR-B38M is similar to previous BCMA-targeting CAR-T cell therapies. Notably, the preliminary results from this exploratory analysis suggest that LCAR-B38M holds significant promise as an effective intervention for RRMM patients.
Non-BCMA CAR-T Targets
BCMA-targeting CAR-T cell therapies have shown encouraging results in RRMM patients. However, long-term follow-up of these patients has revealed that some experience BCMA-negative relapse, similar to CD19-negative relapse observed in other leukemias treated with CD19-targeted CAR-T cells. This has led to the development of alternative CAR-T targets in multiple myeloma.
These novel targets can be developed as single targets or as bispecific CARs in combination with BCMA to achieve more durable responses. Several promising non-BCMA CAR-T targets for MM have emerged, including CD138 (syndecan-1), which is highly expressed on multiple myeloma cells; GPRC5D (G protein-coupled receptor, class C, group 5, member D), a receptor associated with cell proliferation found in multiple myeloma cells; and FcRH5 (Fc receptor homolog 5), a receptor related to the immune response in multiple myeloma cells. While these targets are currently under investigation, they hold significant potential for introducing new treatment options for RRMM patients.
Summary and Future Outlook
Despite the encouraging results achieved thus far, CAR-T cell therapy in multiple myeloma is still in its infancy, and there is room for improvement to unlock its full potential. Innovative strategies to enhance the efficacy, durability, and alleviate tumor cell resistance mechanisms of CAR-T cells are crucial for further improving outcomes. The results of ongoing studies investigating novel CAR-T constructs targeting other multiple myeloma cell antigens are eagerly anticipated. The potential of allogeneic CAR-T cells to overcome many of the manufacturing and logistical limitations of traditional autologous CAR-T cell therapy is also promising. The future prospects of cell therapy are exciting, and we remain optimistic that continued refinement of our evolving treatment paradigms may one day render multiple myeloma a curable disease.
Content Source:找药宝典
