CART T Multiple Myeloma Benefits Relapsed or Refractory Adult Patients
CART T Multiple Myeloma Benefits Relapsed or Refractory Adult Patients
CARsgen Therapeutics recently announced that the National Medical Products Administration (NMPA) of China has officially approved the New Drug Application (NDA) for Zevorcabtagene autoleucel, intended for the treatment of adult patients with relapsed or refractory multiple myeloma.
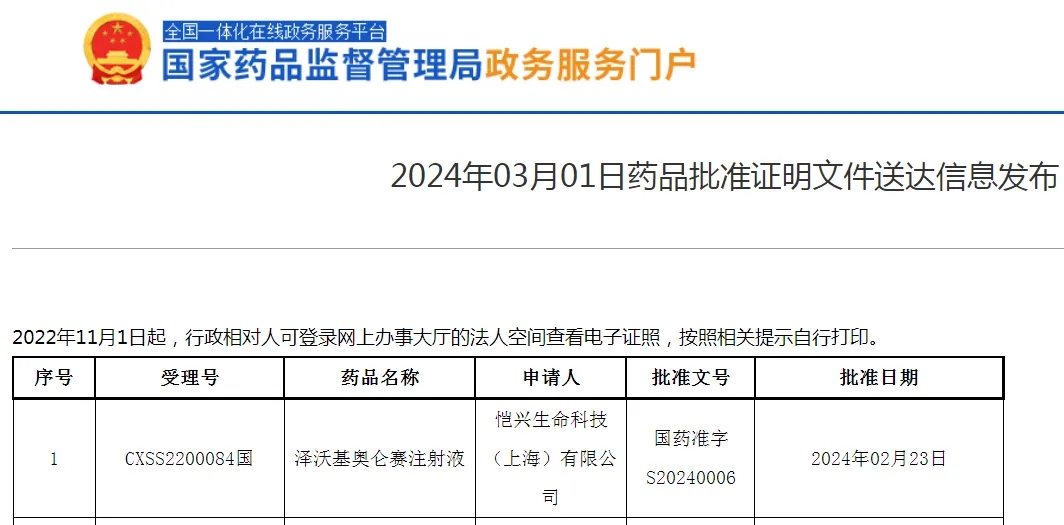
Zevorcabtagene autoloucel approved for listing in China
According to reports, the launch price of CARsgen Therapeutics’ Zevorcabtagene autoleucel is 1.15 million RMB per dose. This pricing was established by the company and its exclusive commercialization partner, Huadong Medicine Co., Ltd., after comprehensively considering the product’s intrinsic value, costs, and commercial sustainability factors.
What is Multiple Myeloma
Multiple myeloma is a refractory malignant plasma cell disease, accounting for approximately 10% of all hematological malignancies. With the acceleration of China’s aging population and the increase in average life expectancy, the number of multiple myeloma patients will continue to rise. According to Frost and Sullivan’s estimates, in 2023, the number of multiple myeloma patients in China is approximately 153,000, with 23,200 new cases. It is projected that the number of multiple myeloma patients in China will increase to 266,300 by 2030.
Introduction to Zevorcabtagene autoleucel
Zevorcabtagene autoleucel is an autologous BCMA-targeted CAR-T cell product generated through lentiviral transduction of T cells. The lentivirus-encoded CAR includes a fully human BCMA-specific single-chain variable fragment (scFv), a human CD8α hinge domain, a CD8α transmembrane domain, a 4-1BB costimulatory domain, and a CD3ζ activation domain. The proprietary fully human scFv possesses high binding affinity and stability.
Five CAR-T Products Approved in China
Zevorcabtagene autoleucel is the fifth CAR-T cell therapy approved for marketing in China, following FOSUN Kite’s Axicabtagene Ciloleucel Injection, JW THERAPEUTICS’ Relmacabtagene autoleucel, IASO Bio’s Equecabtagene Autoleucel, and Juventas’ Inaticabtagene Autoleucel Injection.
| Enterprise | Product Name | China Approval Time | Price |
| FOSUN Kite | Axicabtagene Ciloleucel Injection | June 2021 | 1.2 million yuan / needle |
| JW THERAPEUTICS | Relmacabtagene autoleucel | September 2021 | 1.29 million yuan / needle |
| IASO Bio | Equecabtagene Autoleucel | June 2023 | Rmb1166000 / needle |
| Juventas | Inaticabtagene Autoleucel Injection | November 2023 | 999000 yuan / needle |
| CARsgen Therapeutics | Zevorcabtagene autoleucel | March 2024 | RMB 1.15 million / needle |
Chinese CAR-T Development: From “Following” to “Running Parallel”
In February 2022, Ciltacabtagene Autoleucel, independently developed by the Chinese company Legend Biotech, received approval from the U.S. Food and Drug Administration (FDA) for marketing in the United States.
In February 2024, Ciltacabtagene Autoleucel became the first CAR-T therapy to receive a positive opinion from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) for the second-line treatment of relapsed or refractory multiple myeloma patients.
It can be said that the development of CAR-T technology in China has transitioned from following international trends to running parallel. However, due to the high prices, subsequent promotion remains a challenge for various pharmaceutical companies. Although CAR-T products have participated in the negotiations for inclusion in China’s National Reimbursement Drug List (NRDL) multiple times, none have been successfully added to the list.
Looking at the five products currently on the market, their prices are all above 1 million RMB, while the threshold for inclusion in China’s NRDL is typically between 200,000 and 300,000 RMB. Therefore, the number of patients who can afford these technologies is extremely limited.
Nevertheless, the advancement of technology still represents hope for many patients. As CAR-T technology becomes increasingly mature, and with the support of the Chinese government, it is believed that more patients will be able to access these new technologies in the future.
Assuming you are a professional Chinese-English medical translator, proficient in medical knowledge and Chinese-English translation, capable of accurately translating medical terminology, you are now required to use your expertise to provide a precise English translation of the above article. Medical terminology must be accurately translated, and the meaning must be clear.
Content Source:广西先康达生物
