Latest news on Janssen CAR-T Multiple Myeloma product Ciltacabtagene Autoleucel(Carvykti,LCAR-B38M,JNJ-4528)
Latest news on Janssen CAR-T Multiple Myeloma product Ciltacabtagene Autoleucel(Carvykti,LCAR-B38M,JNJ-4528)
Ciltacabtagene Autoleucel (LCAR-B38M, JNJ-4528, Cilta-cel, Carvykti)
Status: Approved for marketing (FDA)
Date: February 28, 2022
Price: $465,000 per dose
Introduction: The third CAR-T cell product approved in China, Ciltacabtagene Autoleucel is a BCMA CAR-T cell therapy co-developed by Janssen and Legend Biotech.
Indication: Multiple myeloma (relapsed/refractory multiple myeloma in adult patients)
Relevant Clinical Trials and Data:
[CARTITUDE-1 Trial] Overall response rate 98%, with 83% of patients achieving stringent complete response; 18-month progression-free survival rate 66%, 2-year progression-free survival rate 61%; 18-month overall survival rate 81%, 2-year overall survival rate 74%.
Ciltacabtagene Autoleucel Receives Latest FDA Support
Recently, Legend Biotech announced that the U.S. FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 11-0 in favor, determining that Ciltacabtagene Autoleucel (CARVYKTI, cilta-cel) has a favorable benefit-risk profile for the treatment of adult patients with relapsed or lenalidomide-refractory multiple myeloma (R/R MM) after at least one prior line of therapy, including a proteasome inhibitor and an immunomodulatory agent, and who had disease progression on the last regimen and were lenalidomide-refractory.
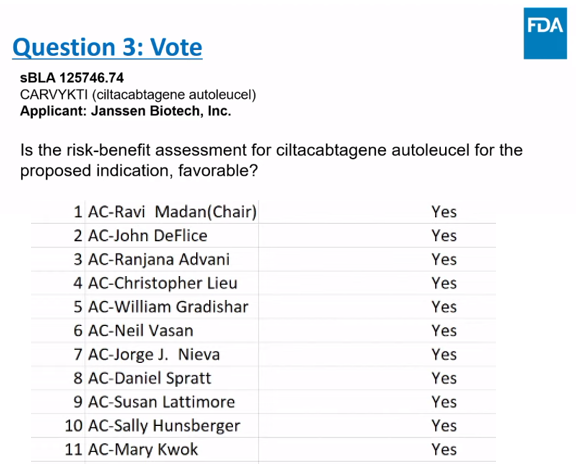
Currently, a supplemental Biologics License Application (sBLA) supported by the CARTITUDE-4 study is under review by the FDA, with a PDUFA date of April 5, 2024.
The advisory committee reviewed the results of CARTITUDE-4 (NCT04181827), the first randomized Phase 3 study evaluating the efficacy and safety of CARVYKTI® compared to Pomalidomide, Bortezomib, and Dexamethasone (PVd) or Daratumumab, Pomalidomide, and Dexamethasone (DPd) in patients with relapsed and lenalidomide-refractory multiple myeloma who had received one to three prior lines of therapy.
The results of the Phase 3 CARTITUDE-4 study were first presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, and these results also supported the recent positive opinion from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) for CARVYKTI® for the treatment of adult patients with relapsed or refractory multiple myeloma, who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), and have had disease progression on the last regimen and are lenalidomide-refractory.
