T cell myeloma,The Lancet Haematology:GPRC5D-Targeted CAR-T Cell Therapy Shows Promising Potential for Relapsed or Refractory Multiple Myeloma
T cell myeloma,The Lancet Haematology:GPRC5D-Targeted CAR-T Cell Therapy Shows Promising Potential for Relapsed or Refractory Multiple Myeloma
OriCAR-017:a first-in-human, single-centre, single-arm, phase 1 trial
Multiple myeloma is a malignant disease characterized by clonal proliferation of plasma cells in the bone marrow. Despite substantial advances in treatment modalities such as systemic chemotherapy, radiation therapy, and hematopoietic stem cell transplantation (HSCT), this disease remains incurable. Chimeric antigen receptor (CAR) T-cell therapy targeting B-cell maturation antigen (BCMA) has shown activity in treating relapsed or refractory multiple myeloma. However, myeloma cells with low or negative BCMA expression can evade BCMA-targeted CAR-T cell therapy and lead to relapse, necessitating the exploration of novel targets.
In February 2023, The Lancet Haematology journal published a study titled “GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial” aimed at evaluating the activity and safety of G protein-coupled receptor family C group 5 member D (GPRC5D) CAR-T cells (named OriCAR-017) in patients with relapsed or refractory multiple myeloma.
The POLARIS trial (NCT05016778) was a first-in-human, single-center, single-arm phase 1 trial of GPRC5D-targeted CAR-T cells conducted at The First Affiliated Hospital, College of Medicine, Zhejiang University, China. Eligibility criteria included adults aged 18-75 years with relapsed or refractory multiple myeloma, an ECOG performance status of 0-2, GPRC5D expression in ≥20% of bone marrow plasma cells or GPRC5D positivity by immunohistochemistry, and prior treatment with at least three regimens, including a proteasome inhibitor, an immunomodulatory drug, and chemotherapy.
In the trial, 9 patients were consecutively assigned to receive a single intravenous infusion of OriCAR-017 at dose levels of 1×10⁶ CAR T cells/kg (3 patients), 3×10⁶ CAR-T cells/kg (3 patients), or 6×10⁶ CAR-T cells/kg (3 patients) in the dose-escalation phase. In the expansion phase, patients received the recommended phase 2 dose. The primary endpoints were safety, maximum tolerated dose, and recommended phase 2 dose. Safety and activity analyses included all patients who received OriCAR-017. The trial has completed, and long-term follow-up is ongoing.
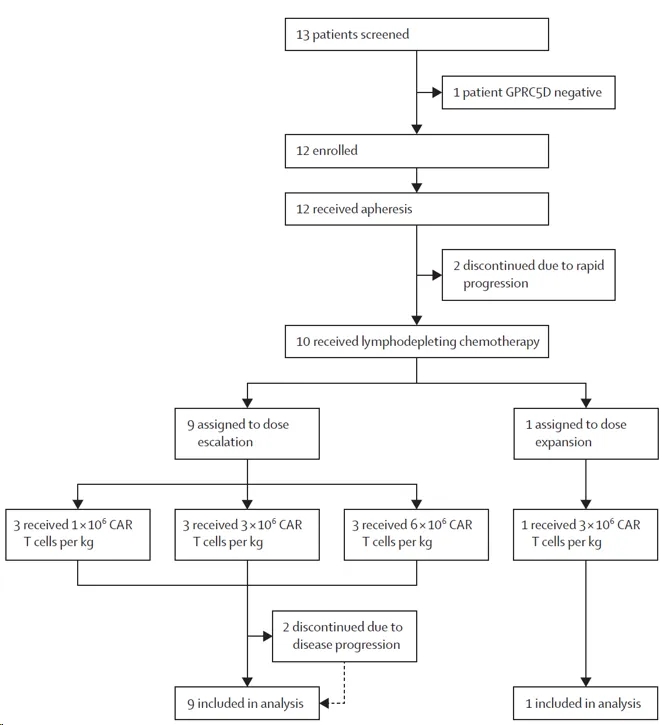
Clinical trial flow chart
01、OriCAR-017 infusion resulted in favorable patient responses
All 10 patients (100%) had an overall response, with 6 (60%) achieving a complete response and 4 (40%) having a very good partial response. Patients with a complete response met the stringent criteria for complete response. All patients were minimal residual disease (MRD) negative at day 28. Serum M-protein concentrations gradually decreased, and clinical responses improved over time (Figure below), with a median time to best response of 3.1 months and a median time to complete response or better of 4.1 months.
Among the 5 patients who relapsed after BCMA-targeted CAR-T cell therapy, 2 achieved a stringent complete response, and 3 had a very good partial response. Four patients had extramedullary disease at baseline, with the largest tumor volume being 70 cm³. PET-CT showed complete resolution of extramedullary disease in 3 patients, and the remaining patient had ongoing shrinkage of extramedullary disease. No serious adverse events or treatment-related deaths occurred.
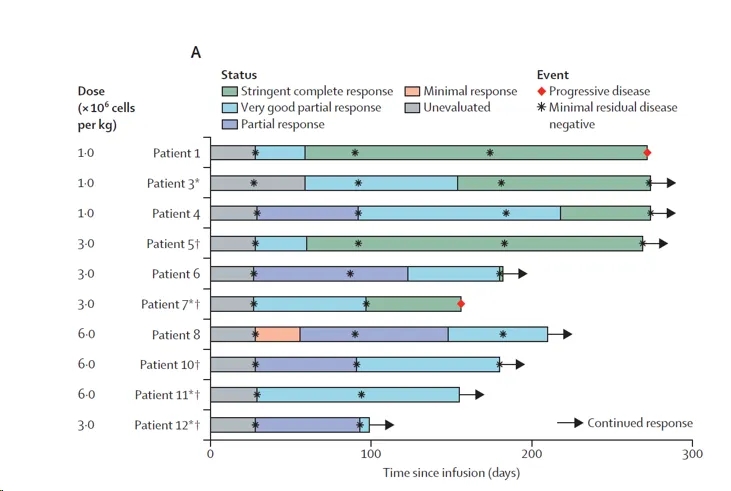
Treatment response of patients after infusion of oricar-017
02、OriCAR-017 infusion resulted in favorable patient survival
With a median follow-up of 238 days, two patients progressed after achieving a stringent complete response. One patient had GPRC5D-positive relapse, with GPRC5D expression in malignant plasma cells increasing from 34.5% at baseline to 35.8% at progression. The other patient had GPRC5D-negative relapse, with GPRC5D expression in malignant plasma cells decreasing from 86.8% at baseline to 6.9% at progression. The remaining 8 patients in remission were minimal residual disease negative, and no deaths occurred. The median progression-free survival was not reached, but the estimated 9-month progression-free survival rate for all patients was 87.5% (Figure below).
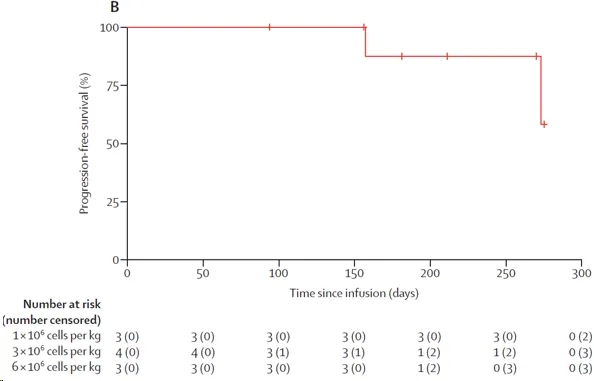
Kaplan Meier curves of progression free survival
03、Persistence of CAR-T cells after infusion
CAR-T cell expansion was detected in all patients after infusion (Figure below), with a median time to maximum CAR-T cell expansion (Cmax) of 10.0 days and a median Cmax of 7930 copies/μl. CAR-T cells exhibited favorable persistence, with 9 (90%) patients having detectable CAR-T cells at 1 month, 7 patients at 3 months, and 4 patients at 6 months.
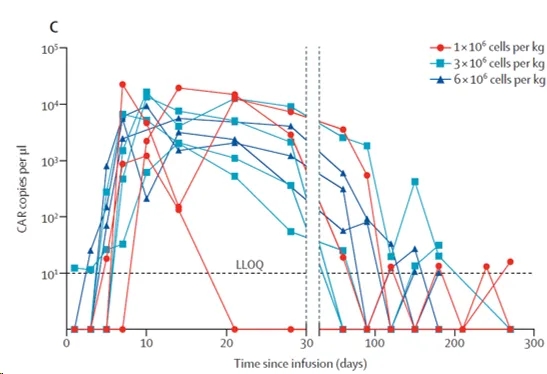
In vivo kinetics of car-t cells
Summary
The results demonstrate that GPRC5D-targeted CAR-T cell therapy for patients with relapsed or refractory multiple myeloma has a favorable safety profile and preliminary activity. The on-target and off-target toxicities observed with GPRC5D targeting in this trial were manageable, suggesting that GPRC5D has the potential to be an effective immunotherapeutic target in multiple myeloma. These findings provide a foundation for subsequent phase 2 studies to further validate the efficacy and safety of GPRC5D-targeted CAR-T cells in multiple myeloma.
Future research is needed to explore the sequence analysis of BCMA and GPRC5D targets for immunotherapy and to investigate whether combining these two targets could provide enhanced and more durable responses.
References:Zhang, Mingming et al. “GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial.” The Lancet. Haematology vol. 10,2 (2023): e107-e116. doi:10.1016/S2352-3026(22)00372-6
Content Source:上海细胞治疗工程技术研究中心
