Abecma and Carvykti BCMA CAR-T Therapies Poised for Frontline Use, Market Size Expected to Expand Six-fold
Abecma and Carvykti BCMA CAR-T Therapies Poised for Frontline Use, Market Size Expected to Expand Six-fold
March 15,2024,the FDA Oncologic Drugs Advisory Committee (ODAC) held a full-day meeting to review the benefit/risk profiles of Abecma from BMS/Bluebird Bio and Carvykti from Johnson & Johnson/Legend Biotech, two BCMA CAR-T therapies for frontline treatment.
Eleven experts participated in the review. The results showed that all experts unanimously acknowledged that the benefits of Carvykti outweighed the risks, while the vote for Abecma was 8:3 in favor of its benefits outweighing the risks.
The ODAC’s assessment is a non-binding recommendation, and the FDA is not obliged to follow it, but it rarely goes against such recommendations.
The industry’s focus on this event is twofold:
1. Since the approval of the first CAR-T therapy, these treatments have been used as later-line therapies. If the indications can be successfully moved to earlier lines, the number of covered patients is expected to increase several times, potentially allowing a single product to achieve peak sales of tens of billions of dollars.
2. The FDA is currently investigating a new safety signal concerning secondary T-cell lymphoma associated with CAR-T therapies and has added a black box warning for secondary malignancies to all CAR-T therapies. Against this backdrop, the expert discussions will help the industry observe the FDA’s stance on the risk assessment of CAR-T therapies.

Photo source: Fierce Pharma
Market Size Expected to Expand Six-fold
Carvykti is a flag bearer for China’s innovative drugs going global. In March 2022, Carvykti was first approved in the US for the fifth-line treatment of relapsed/refractory multiple myeloma. It has since been approved in the European Union and Japan.
Abecma is also a CAR-T therapy targeting BCMA (B-cell maturation antigen) and was approved in 2021 for the fifth-line treatment of the same disease.
In June 2023, the latest clinical trial data for Carvykti was presented at the ASCO meeting. In over 400 multiple myeloma patients who had received 1-3 prior lines of therapy and were refractory to lenalidomide, Carvykti reduced the risk of disease progression or death by 74% compared to standard therapy.
The latest clinical trial data for Abecma showed that it could reduce the risk of disease progression or death by 51% in multiple myeloma patients who had developed triple-drug resistance.
In terms of patient population coverage, only 15% of patients receive fourth-line or later treatment. Carvykti’s study extended to patients who had received 1-3 lines of therapy, meaning that the coverage expanded to 95%, representing a six-fold increase in market potential.
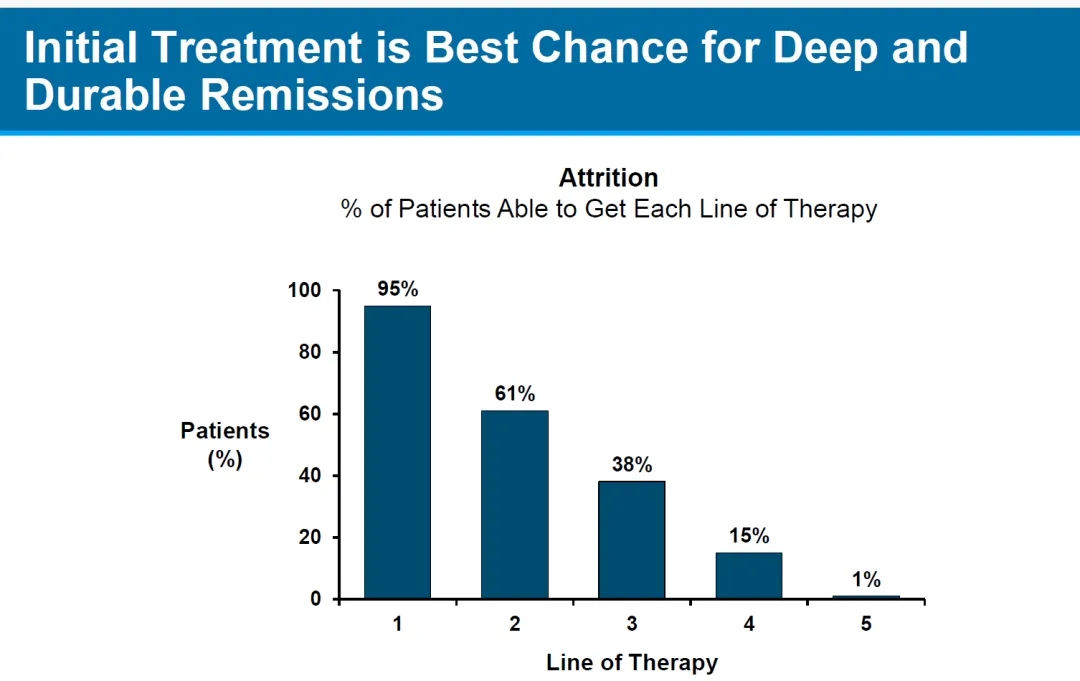
In 2023, its first year of commercialization, Carvykti achieved global sales of as high as $5 billion, with a target peak sales of over $50 billion. If the frontline indication is approved, achieving this target should not be an issue, although the production capacity constraint needs to be addressed.
“Black Box Warning” Notwithstanding, Experts Believe Long-term Benefits Outweigh Risks
The most significant controversy surrounding this approval is the safety issue associated with CAR-T therapies.
By the end of 2023, the FDA stated that it had received 19 reports of secondary malignancies following CAR-T therapy since the first approval in 2017, with 14 of these reports coming from the FDA’s self-reporting adverse event system, FAERS.
In the two phase III clinical trials for Carvykti and Abecma, the FDA pointed out that in the first few months after trial initiation, the mortality rates were higher in the CAR-T cell therapy arms compared to the standard drug combination arms. Of particular concern was that these early deaths were not due to disease progression but were associated with adverse events.
The ODAC voting results indicate that despite the FDA’s concerns, experts unanimously believe that the long-term benefits of these drugs outweigh the risks, and the agency’s attention should not be focused on those statistically insignificant initial events. Instead, the risks should be viewed in the context of Carvykti’s statistically significant benefits over a longer period.
Reports suggest that some experts at the drug review meeting noted that the fact that some patients died while waiting to receive Carvykti was an important factor contributing to the trial deaths.
However, the voting numbers suggest that the FDA seems more concerned about the safety of Abecma (8:3) than Carvykti (11:0).
The FDA is expected to make its final decision on Carvykti’s application by April 5.
In Europe, the prospects for approving Abecma and Carvykti as early treatment options are optimistic. In January 2023, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended approving Abecma for the treatment of relapsed or refractory multiple myeloma (RR MM) in patients who have received at least two prior therapies. Shortly after, Carvykti received a similar recommendation from the CHMP in February.
Potential of CAR-T Development, To Be Continued
In addition to treating blood cancers, CAR-T cell therapies also hold promise for “curing” autoimmune diseases.
On February 22, a team of scientists from the Friedrich-Alexander University Erlangen-Nuremberg in Germany published a paper in the prestigious New England Journal of Medicine. They detailed the cases of 15 individuals with severe autoimmune diseases who experienced disease remission or significant symptom improvement after receiving CAR-T cell therapy.

These patients suffered from difficult-to-treat lupus, inflammatory myositis, or systemic scleroderma. After receiving CD19-targeted CAR-T treatment, a median follow-up of 15 months showed sustained disease remission or substantial symptom reduction, allowing them to discontinue all immunosuppressive and anti-inflammatory medications. This result is a milestone in the treatment of autoimmune diseases.
The research findings were initially presented at a medical conference in December of the previous year, but the NEJM paper provided a more detailed account of the study results.
Apart from the remarkable efficacy, the trial also demonstrated an excellent overall safety profile for CAR-T cell therapy in autoimmune diseases, with only mild toxicities observed.
CAR-T can induce a “reset” of the patient’s autoimmune disease. In the months following treatment, the participants’ B-cell networks were reconstituted, and the levels of pathogenic “autoantibodies” associated with inflammatory diseases decreased. After B-cell replenishment, participants showed no signs of relapse for up to two years.
Although the German study was small-scale and lacked a placebo control, its results have influenced numerous pharmaceutical companies. Since 2022, biotech and pharmaceutical companies have initiated around a dozen clinical trials of CAR-T cell therapies for lupus. At least six companies have obtained FDA approval to initiate clinical trials.
Companies are also evaluating CAR-T therapies for other autoimmune diseases, such as myasthenia gravis and multiple sclerosis.
Autoimmune diseases have been dubbed “the undying cancer,” with no cure available and patients requiring lifelong treatment. By transitioning from “cancer treatment” to “autoimmune disease treatment,” the further development of CAR-T therapies opens up new potential. Major pharmaceutical companies like Novartis and AstraZeneca are taking action.
Reference Material
1、https://www.fiercepharma.com/pharma/fda-advisers-back-carvykti-earlier-multiple-myeloma-treatment-despite-early-death-concerns
2、https://mp.weixin.qq.com/s/6GaE5XV1iK8VFMbZkEY_Tw
Content Source:MedTrend医趋势
