March 1, 2022 FDA Approval Carvykti for the treatment of relapsed/refractory multiple myeloma
March 1, 2022 FDA Approval Carvykti for the treatment of relapsed/refractory multiple myeloma
FDA Approves Carvykti for Marketing
On March 1, 2022, Genscript Biotech officially announced that its subsidiary Legend Biotech’s CAR-T product Carvykti (Ciltacabtagene autoleucel) received FDA approval. Carvykti is a BCMA-targeted CAR-T therapy, approved this time for the treatment of relapsed/refractory multiple myeloma.

Carvykti® (Cilta-cel, Ciltacabtagene autoleucel) is Legend Biotech’s first approved product to market
This approval was primarily based on the pivotal results from the Phase 1b/2 CARTITUDE-1 study. The latest data showed that Cilta-cel demonstrated an overall response rate of up to 98% in patients with relapsed or refractory multiple myeloma who had previously received four or more lines of therapy (including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody).

On February 28, 2022 local time, Legend Biotech (NASDAQ: LEGN) officially announced in Somerset, New Jersey, USA, that its independently developed cell therapy product Ciltacabtagene autoleucel (trade name: Carvykti®, common name ciltacabtagene autoleucel, abbreviated as Cilta-cel) has been approved by the US FDA for marketing to treat patients with relapsed or refractory multiple myeloma (R/R MM) who have received four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. Legend Biotech signed an exclusive global licensing and collaboration agreement with Janssen in December 2017 to develop and commercialize Carvykti®.
(Image source: Endpoints News)
Not only does this dispel the gloom over China’s drug companies going abroad after 2.11, fighting a brilliant “comeback battle”, but it also means that China has officially reached the world’s leading position in the field of “cell therapy”:
This is the first CAR-T therapy designed and developed by a Chinese pharmaceutical company to receive FDA approval;
This is the second anti-cancer new drug invented by a Chinese company to receive FDA approval, more than two years after the previous one (BeiGene’s Zanubrutinib);
This is the second BCMA-targeted CAR-T therapy approved for marketing.
About Carvykti
Carvykti® is a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T-cell therapy with two BCMA-targeting single domain antibodies, administered as a one-time infusion at a recommended dose of 0.5–1.0 × 106 CAR-positive viable T cells per kg body weight. In the pivotal CARTITUDE-1 study, 97 R/R MM patients achieved early, deep, and durable responses, with an overall response rate (ORR) of 98% (95% CI: 92.7-99.7), and 78% of patients achieved a stringent complete response (sCR; 95% CI: 68.8-86.1). At a median follow-up of 18 months, the median duration of response (DOR) was 21.8 months (95% CI: 21.8–not estimable).
Carvykti® is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Carvykti® REMS. Carvykti® has a Boxed Warning for cytokine release syndrome (CRS), neurologic toxicities, parkinsonism and Guillain-Barré syndrome, hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS), and prolonged and recurrent cytopenia. Warnings and Precautions include prolonged and recurrent cytopenia, infections, hypogammaglobulinemia, hypersensitivity reactions, secondary malignancies, and effects on ability to drive and use machines. The most common adverse reactions (≥20%) are fever, CRS, hypogammaglobulinemia, hypotension, musculoskeletal pain, fatigue, infection of unspecified pathogen, cough, chills, diarrhea, nausea, encephalopathy, decreased appetite, upper respiratory tract infection, headache, tachycardia, dizziness, dyspnea, edema, viral infections, coagulopathy, constipation, and vomiting.
Dr. Ying Huang, CEO and CFO of Legend Biotech, commented:
“Multiple myeloma remains an incurable disease, and patients who have received multiple prior therapies face poor prognoses and limited treatment options. The approval of Carvykti® is a significant milestone for Legend Biotech, as it is our first product approval since the company’s founding. More importantly, this therapy has the potential to be a new effective treatment option for patients in need of durable treatment-free remissions. This is the first of many cell therapies we plan to bring to patients as we continue to advance our robust pipeline across various disease areas.”
Multiple myeloma is a cancer that forms in plasma cells, a type of white blood cell that normally produces antibodies. Most patients eventually relapse and become resistant to standard therapies, including immunomodulatory agents, proteasome inhibitors, and anti-CD38 antibodies, leaving them with limited effective treatment options.
“For most patients with multiple myeloma, the treatment journey is one of continuous cycles of remission and relapse, with fewer patients achieving deep and durable responses after later lines of therapy,” said Sundar Jagannath, M.D., Professor of Medicine/Multiple Myeloma and Director of the Center of Excellence for Multiple Myeloma at The Tisch Cancer Institute at Mount Sinai, and a lead study investigator of CARTITUDE-1. “This is what makes the results from the CARTITUDE-1 study with Carvykti® so exciting, as they show that heavily pre-treated patients with this advanced disease were able to achieve deep and durable responses with extended treatment-free remissions. This approval has the potential to address a significant unmet need for these patients.”
As a personalized therapy, the administration of Carvykti® requires extensive training, preparation, and certification to ensure an optimal patient experience. Beginning in 2022, Legend and Janssen will initiate a phased approach to introducing certified treatment centers, expanding manufacturing capacity, and increasing availability of Carvykti® across the U.S. to ensure reliable and timely product delivery to hematologists and their patients.
About Carvykti® (Cilta-cel, Ciltacabtagene autoleucel)
Carvykti is a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T-cell therapy that uses a patient’s own T cells that are genetically engineered to target and eliminate BCMA-expressing cells. BCMA is expressed on the surface of multiple myeloma cells, as well as late-stage B cells and plasma cells. Carvykti® has two BCMA-targeting single domain antibodies designed to confer high avidity against human BCMA-expressing cells. Upon binding to BCMA on the target cell, the CAR promotes T-cell activation, expansion, and elimination of the target cell.
In December 2017, Janssen entered into an exclusive worldwide license and collaboration agreement with Legend Biotech to develop and commercialize Carvykti®. In April 2021, Legend Biotech announced the submission of a Marketing Authorization Application to the European Medicines Agency seeking approval of Cilta-cel for the treatment of patients with relapsed or refractory multiple myeloma. In addition to being granted Breakthrough Therapy Designation in the U.S. in December 2019, Cilta-cel also received Breakthrough Therapy Designation in China in August 2020 and was granted Orphan Drug Designation by both the U.S. FDA and European EMA in February 2019 and February 2020, respectively.
Ciltacabtagene autoleucel (Cilta-cel) demonstrates promising clinical data
The FDA’s approval was based on the results of the pivotal phase Ib/II CARTITUDE-1 study, which evaluated the efficacy and safety of cilta-cel in patients with relapsed/refractory multiple myeloma. Of the 97 enrolled subjects, 99% were refractory to the last line of treatment, and 88% had previously received at least three lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody, without response. The results showed that at a median follow-up of 12.4 months, the independent review committee-assessed objective response rate (ORR) was 97%, including 67% stringent complete responses (sCRs), and 92.8% of patients achieved a very good partial response (VGPR) or better. The incidence of grade ≥3 cytokine release syndrome (CRS) was 4%, and the incidence of grade ≥3 neurotoxicity was 9%.
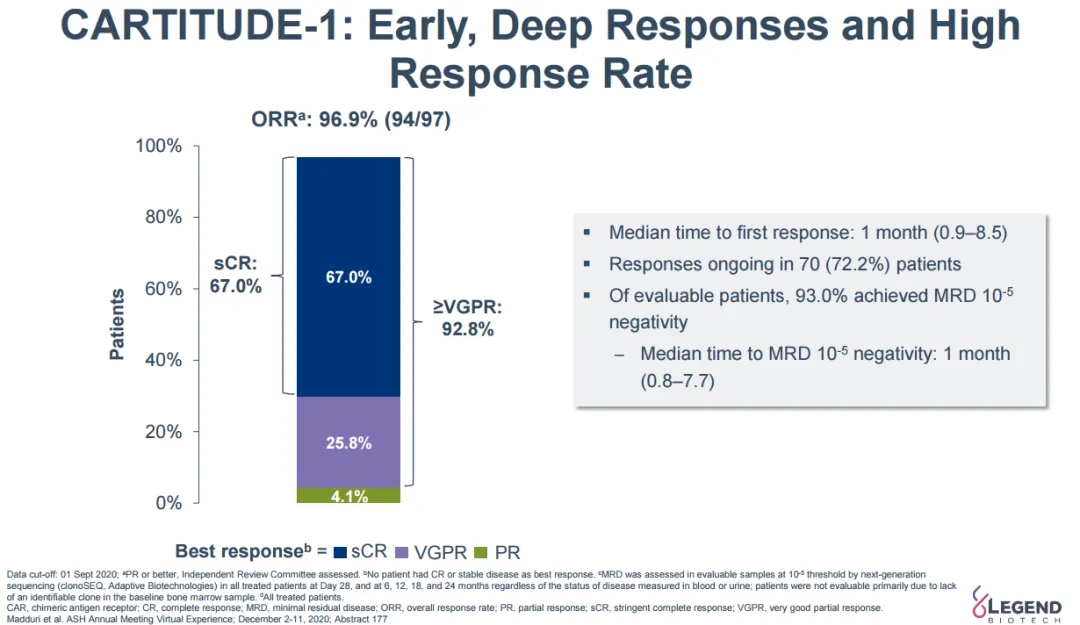
CARTITUDE-1 study 12.4-month follow-up data (Legend Biotech website)
At the most recent 2021 ASCO annual meeting, Legend updated the 18-month median follow-up data from the CARTITUDE-1 study, showing that patients treated with Ciltacabtagene autoleucel achieved an ORR of 97.9%.
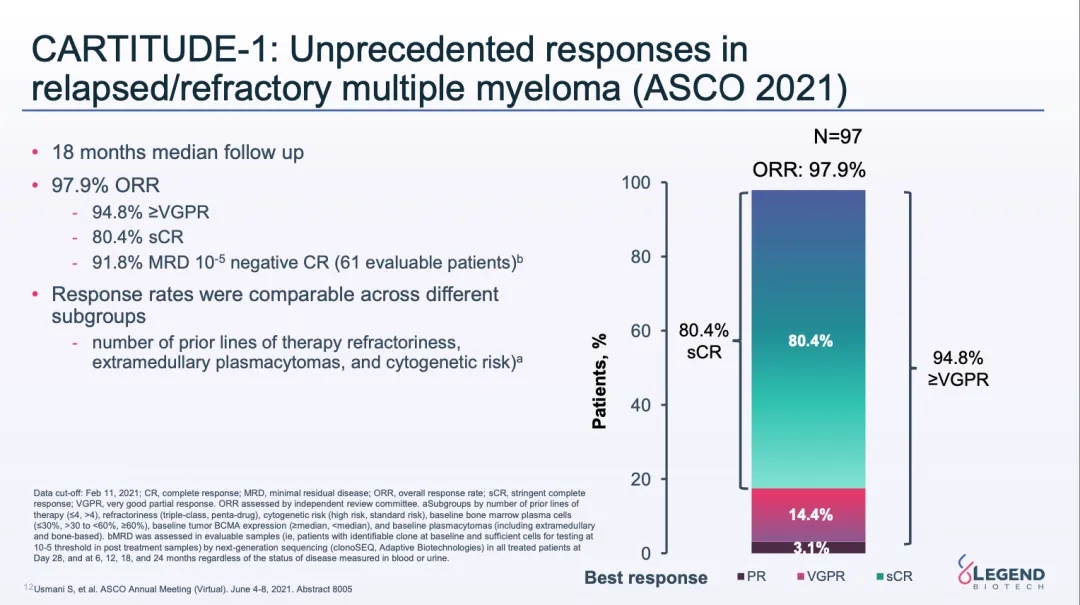
CARTITUDE-1 study 18-month follow-up data (Source: 2021 ASCO)
Preliminary data from Cohort A of the CARTITUDE-2 study showed an ORR of 95% in MM patients who had previously received one to three lines of therapy, with 75% achieving sCR/CR.
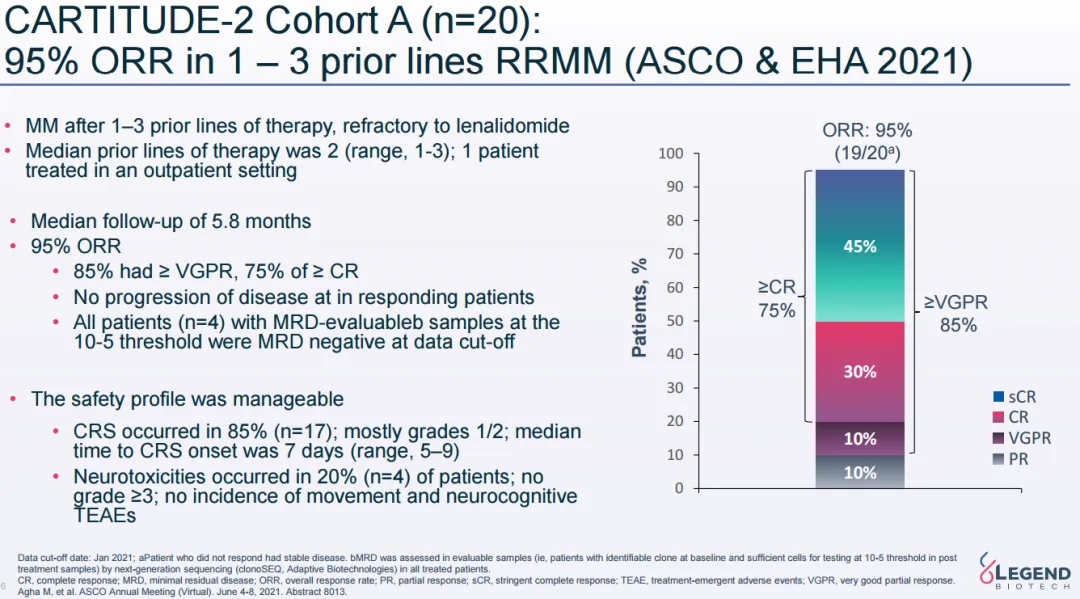
CARTITUDE-2 study follow-up data (Source: 2021 ASCO)
Compared to other CAR-T products in the same class, Ciltacabtagene autoleucel not only has a unique drug structure but also a significantly lower clinical dosing regimen, resulting in high safety and exceptional efficacy.
About the CARTITUDE-1 study
CARTITUDE-1 is an ongoing Phase 1b/2, open-label, single-arm, multi-center study evaluating the safety and efficacy of Carvykti® in adults with relapsed or refractory multiple myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor (PI), an immunomodulatory agent (IMiD), and an anti-CD38 monoclonal antibody. Of the 97 patients enrolled in the study, 99% were refractory to the last line of treatment, and 88% were triple-class refractory, meaning they had no response or had stopped responding to an IMiD, a PI, and an anti-CD38 monoclonal antibody. The ongoing CARTITUDE-1 study is evaluating the long-term efficacy and safety of Carvykti®, with the most recent two-year follow-up results presented at the 2021 ASH annual meeting.
About Multiple Myeloma
Multiple Myeloma (MM) is considered an incurable blood cancer that arises from the dysregulated proliferation of plasma cells in the bone marrow. According to estimates from the American Cancer Society, more than 34,000 new cases of multiple myeloma will be diagnosed in the United States in 2022, and more than 12,000 people will die from the disease. While some patients with multiple myeloma have no apparent symptoms, most are diagnosed due to the presence of symptoms, which can include bone disease, low blood cell counts, hypercalcemia, kidney problems, or infections. Although treatment may result in remission, patients are likely to relapse. Patients who relapse after treatment with standard therapies, including proteasome inhibitors, immunomodulatory agents, and anti-CD38 monoclonal antibodies, face a poor prognosis and limited treatment options.
Content Source:汇聚南药
