Understanding CAR-T Cells and Multiple Myeloma Therapy in One Read
Understanding CAR-T Cells and Multiple Myeloma Therapy in One Read
Many lymphoma and leukemia patients are familiar with CAR-T cell therapy, but for multiple myeloma patients, CAR-T cell therapy is still a novel concept. What exactly is CAR-T cell therapy, and what can it bring to multiple myeloma patients?
What is CAR-T Cell Therapy
CAR-T is a chimeric antigen receptor T-cell therapy, a form of cellular immunotherapy, which is a new type of cancer treatment technology besides surgery, radiotherapy, and chemotherapy. It originates from the patient’s own T lymphocytes. Healthy T lymphocytes are collected from the patient, engineered ex vivo, and cultured into cytotoxic T cells specifically targeting tumor antigens—these are the CAR-T cells.
After receiving the CAR-T cell infusion, the CAR-T cells will exert their tumor-killing and clearance effects within the body. Currently, in cellular immunotherapy, CAR-T therapy is the most targeted and effective form of specific cellular immunotherapy for eliminating tumors.
The Process of CAR-T Cell Therapy
1. Selecting Soldiers: First, doctors “select” T lymphocytes from the patient’s body. Many myeloma patients have undergone or are familiar with autologous stem cell transplantation. The process of collecting lymphocytes is similar to collecting stem cells but simpler, involving the collection of normal peripheral blood, followed by ex vivo culture and expansion of the collected T lymphocytes. Some patients say it feels “just like drawing blood.”
2. Modifying the “Radar”: After expansion, the T lymphocytes still cannot recognize the patient’s tumor cells and need to be equipped with a “radar” to become T cells that can identify myeloma cells—these are the CAR-T cells.
3. Preconditioning Chemotherapy: After collecting T lymphocytes from the patient, the patient undergoes preconditioning chemotherapy to temporarily suppress the immune system, preventing the infused CAR-T cells from being rejected by the body’s normal immune system in the short term.
4. CAR-T Cell Infusion: After chemotherapy, the CAR-T cells are infused and begin specifically eliminating tumor cells.
The Efficacy of CAR-T Cell Therapy
CAR-T cell therapy has a history of six to seven years, initially applied to acute lymphoblastic leukemia. Its tumor cell clearance ability is very strong. For patients with relapsed or refractory B-cell acute lymphoblastic leukemia, CD19-CAR-T cell therapy can achieve a complete remission rate of 80%-90%; for patients with relapsed or refractory B-cell lymphoma, CD19-CAR-T cell therapy can achieve a complete remission rate of 40-60%, significantly better than conventional chemotherapy.
How effective is CAR-T cell therapy for multiple myeloma? For multiple myeloma, there is a special target called BCMA that can be recognized by CAR-T cells. BCMA-targeted CAR-T cells have shown excellent effects for relapsed or refractory multiple myeloma, with complete and very good partial remission rates of 50%-90%.
Of course, in addition to the BCMA target, there are many potential targets for CAR-T cell therapy in multiple myeloma. These targets may be more suitable for patients who have failed BCMA-CAR-T therapy or require further treatment, but currently, BCMA remains the most commonly used and effective target.
When Should CAR-T Cell Therapy Be Considered
Currently, CAR-T cell therapy is primarily applied to patients with relapsed or refractory disease after standard treatment failure, i.e., when the mainstream treatment regimen is ineffective. For example, patients diagnosed with relapsed or refractory multiple myeloma after multiple lines of treatment (including Bortezomib and Lenalidomide).
Additionally, the following conditions can create favorable conditions for CAR-T cell therapy:
●The patient’s physical condition is good, and they can tolerate CAR-T cell therapy and its adverse effects.
●The patient’s peripheral blood can yield a sufficient quantity and quality of T lymphocytes for collection.
●Patients preparing for CAR-T cell therapy should not have active infections, as the adverse effects of immunotherapy would be exacerbated by an infection.
●The patient’s tumor pathology or minimal residual disease testing shows expression of the corresponding target, which may guide the subsequent efficacy to some extent.
●It is noteworthy that if the patient has central nervous system issues, CAR-T cell therapy should be considered very cautiously.
Currently, the biggest challenge for multiple myeloma patients receiving CAR-T therapy is that patients often receive treatment at a late stage when the tumor burden is heavy. If CAR-T therapy could be administered earlier, patients would have a higher success rate and fewer adverse effects. If high-risk multiple myeloma patients could be identified early, CAR-T cell therapy could be considered earlier, benefiting patients sooner.
Therefore, it is recommended that patients undergo risk factor testing, including FISH and karyotype analysis, at initial diagnosis and during disease progression to aid physicians in assessing the disease and selecting appropriate treatment measures.
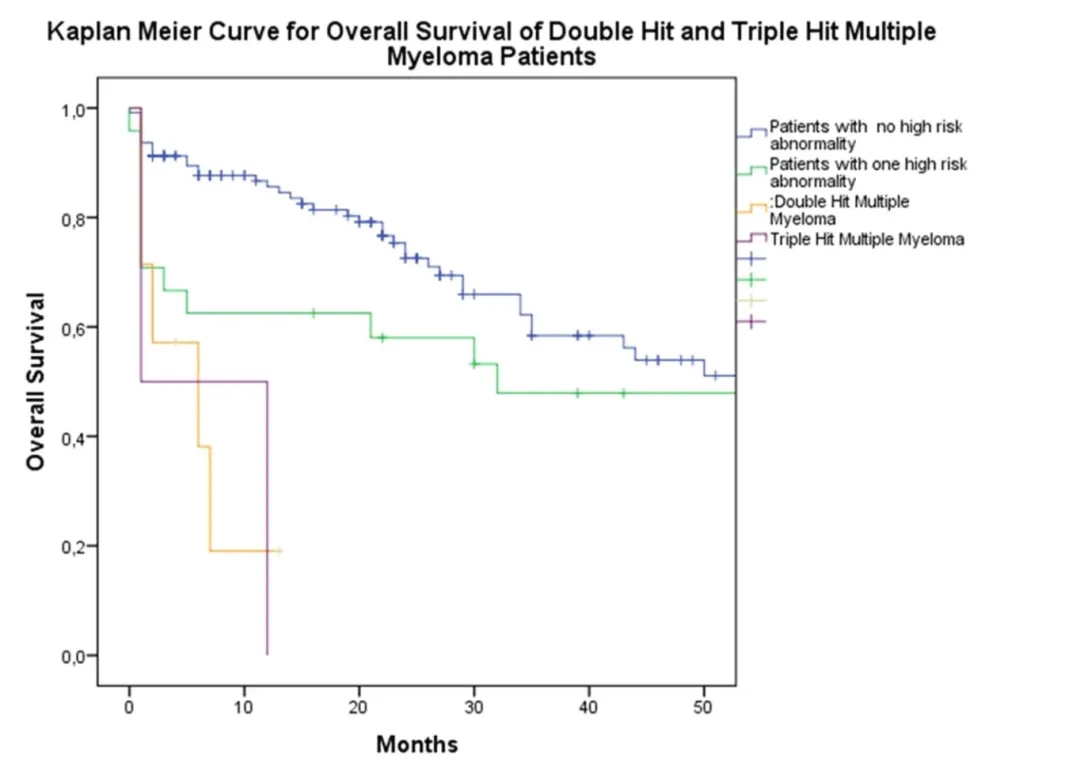
High-risk multiple myeloma patients do not respond well to chemotherapy and should proceed to immunotherapy or transplantation early.
Furthermore, for patients with relapsed or refractory multiple myeloma who have failed multiple lines of treatment, in addition to BCMA-CAR-T cell therapy, other treatment options include CD38 monoclonal antibodies, allogeneic stem cell transplantation, and PD-1 inhibitors.
Adverse Effects of CAR-T Cell Therapy
Adverse effects are a concern for many people. Will the adverse effects of CAR-T cell therapy be severe? What are the potential adverse effects? Based on experience, the most common adverse effect after CAR-T cell therapy is fever, which occurs because the CAR-T cells release cytokines during the process of killing tumor cells, leading to fever in patients.
Concept of cytokine storm
(Source: Jennifer R. Tisoncik et al.)
Another adverse effect is bone marrow suppression, as patients undergo chemotherapy before CAR-T cell therapy, which may cause some degree of bone marrow suppression. Additionally, the incidence of adverse effects such as edema and respiratory distress is relatively low.
If BCMA is Not Expressed, Are There Other Treatment Options
At the 2019 American Society of Hematology Annual Meeting, many scholars discussed this question. Currently, under investigation for relapsed or refractory multiple myeloma CAR-T cell therapy targets include CD19, light chains, CD38, CD138, SLAMF7, GPRC5D, and NKG2D, among others. However, these targets all have potential issues, such as insufficient expression levels or overly broad expression in other cells, which could increase the adverse effects of treatment. Although still in the exploratory stage, these treatment options are worth anticipating.
Content Source:医脉通血液科
