Ciltacabtagene Autoleucel (Carvykti) Approved by FDA for Second-Line Treatment of Relapsed or Refractory Multiple Myeloma
Ciltacabtagene Autoleucel (Carvykti) Approved by FDA for Second-Line Treatment of Relapsed or Refractory Multiple Myeloma
Johnson & Johnson and Legend Biotech’s jointly developed chimeric antigen receptor (CAR) T-cell therapy Carvykti (ciltacabtagene autoleucel, cilta-cel) has been approved by the U.S. FDA for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have previously received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), and are refractory to lenalidomide.
According to the press release, Carvykti is the first FDA-approved B-cell maturation antigen (BCMA)-directed therapy for the treatment of RRMM patients in the second-line or later setting, encompassing CAR-T cell therapy, bispecific antibodies, and antibody-drug conjugates (ADCs).

This approval was primarily based on data from the CARTITUDE-4 study, an international, randomized, open-label phase 3 study evaluating the efficacy and safety of Carvykti compared to standard of care (SOC) regimens in adults with lenalidomide-refractory RRMM who had received 1-3 prior lines of therapy. The SOC regimens in the study included Pomalidomide, Bortezomib, and Dexamethasone (PVd) or Daratumumab, Pomalidomide, and Dexamethasone (DPd).
According to the FDA’s ODAC meeting materials, Carvykti demonstrated a statistically significant improvement in progression-free survival (PFS) compared to the SOC arm, as assessed by an independent review committee (IRC) in the CARTITUDE-4 trial (HR=0.41, 95% CI: 0.30-0.56, p<0.0001).
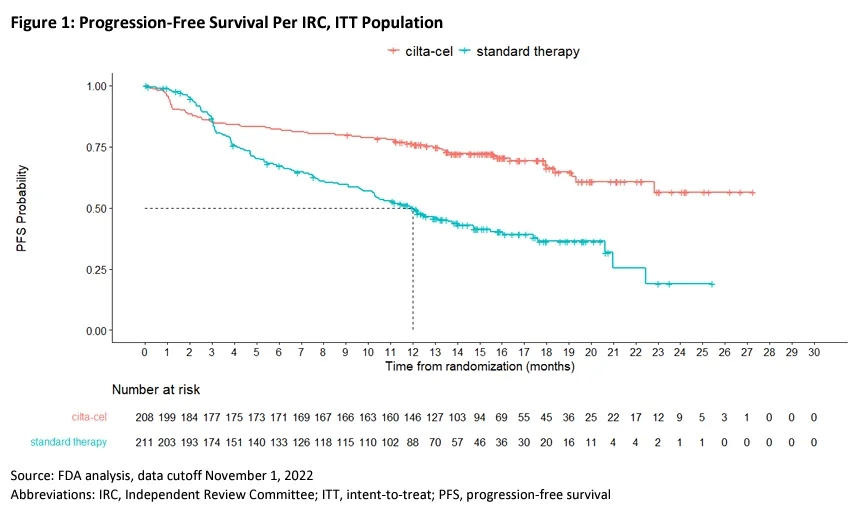
▲FDA’s analysis of PFS in the CARTITUDE-4 trial (Image source: Reference [2])
In the second interim analysis, the median overall survival (OS) was not reached in the Carvykti arm, while the estimated median OS for the SOC arm was 26.7 months (HR=0.78, 95% CI: 0.51-1.20).
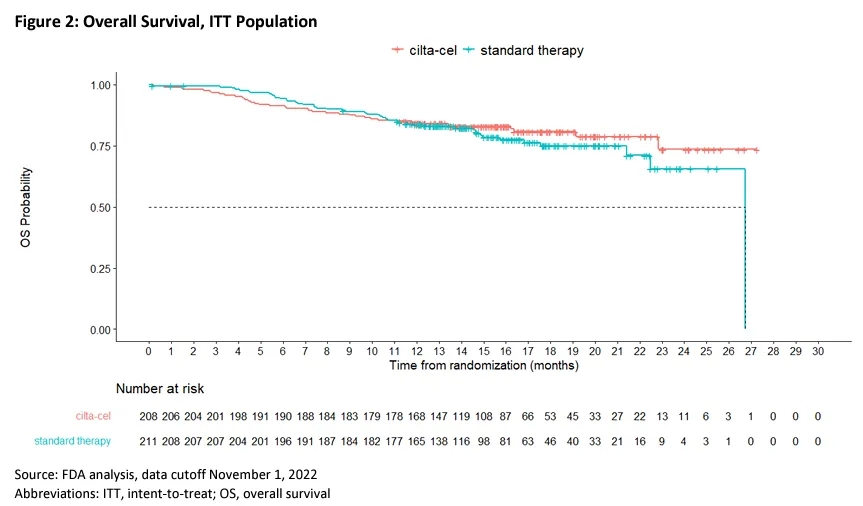
▲FDA’s analysis of OS in the CARTITUDE-4 trial (Image source: Reference [2])
Last month, the FDA’s Oncologic Drugs Advisory Committee (ODAC) unanimously voted 11-0 to recommend approval of Carvykti for the treatment of adult patients with relapsed or refractory multiple myeloma.
Carvykti is a BCMA-directed CAR-T cell therapy that uses a chimeric antigen receptor to modify a patient’s own T cells to recognize and eliminate cells expressing BCMA. BCMA is primarily expressed on the surface of malignant multiple myeloma B cells, late-stage B cells, and plasma cells. The CAR protein in Carvykti contains two BCMA-targeting single-domain antibodies, providing high avidity for BCMA-expressing cells. Upon binding to BCMA-expressing cells, the CAR promotes T-cell activation, expansion, and subsequent elimination of the target cells.
In December 2017, Johnson & Johnson and Legend Biotech entered into a worldwide exclusive license and collaboration agreement to develop and commercialize Carvykti. It received FDA approval in February 2022 and conditional marketing authorization from the European Union in May 2022. In September 2022, it was approved by Japan’s MHLW for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy.
Assuming you are a professional medical translator proficient in medical knowledge and English-Chinese translation, capable of accurately translating medical terminology, please use your expertise to provide an accurate English translation of the above article, ensuring precise translation of medical terms and clear semantics.
Reference Material
[1] Legend Biotech’s CARVYKTI® (ciltacabtagene autoleucel) Becomes the First and Only BCMA-Targeted CAR-T Cell Therapy Approved by the FDA for Second-Line Treatment of Multiple Myeloma. Retrieved April 6, 2024 from https://www.businesswire.com/news/home/20240405663733/en
[2] FDA Briefing Document. Retrieved March 15, 2024 https://www.fda.gov/media/176986/download
Content Source:干细胞之家网
