ddBCMA CAR-T (now named Anitocabtagene Autoleucel, Anito-cel) Demonstrates Durable Efficacy with 100% Overall Response Rate
ddBCMA CAR-T (now named Anitocabtagene Autoleucel, Anito-cel) Demonstrates Durable Efficacy with 100% Overall Response Rate
At the end of 2023, Arcellx, an immune cell therapy company, revealed the latest clinical data from the Phase 1 extension study of its cell therapy product, anitocabtagene autoleucel (anito-cel, previously known as CART-ddBCMA), for the treatment of relapsed or refractory multiple myeloma. According to the International Myeloma Working Group (IMWG) criteria, all evaluable patients achieved a 100% overall response rate (ORR), with deep and durable responses observed in patients with high-risk features.

The latest disclosed data continued to demonstrate robust long-term responses, with median duration of response, progression-free survival (PFS), and overall survival not yet reached at the data cutoff. These data, with a median follow-up of 26.5 months post-anito-cel infusion, were reported as an oral presentation at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, with a data cutoff date of October 15, 2023.
As of October 15, 2023, a total of 38 patients were evaluable for efficacy and safety assessments, with a median follow-up of 26.5 months post-treatment. These evaluable patients included the dose-escalation cohorts at the first dose level (1 billion CAR+ T cells, n=6) and the second dose level (3 billion CAR+ T cells, n=6), as well as the dose expansion cohort at the 1 billion CAR+ T cell dose level (n=26).
The interim results from the Phase 1 study of anito-cel (data cutoff date: October 15, 2023) demonstrated deep and durable responses in patients with high-risk features.
Specific trial results are as follows:
According to IMWG criteria, the ORR in all evaluable patients was 100%.
Among the 38 evaluable patients, 29 achieved a complete response (CR) or stringent CR (sCR) (>CR rate, 76%).
Among the 38 evaluable patients, 35 achieved a very good partial response (VGPR) or better (>VGPR rate, 92%).
Of the 28 patients evaluable for minimal residual disease (MRD) testing, 25 (89%) were MRD-negative at a sensitivity of at least 10-5.
As of the October 15, 2023, data cutoff, the median duration of response, PFS, and overall survival were not yet reached. While the median PFS was not reached, the Kaplan-Meier estimate of PFS was 28 months at the data cutoff.
The Kaplan-Meier estimated rates of PFS at 6, 12, 18, and 24 months were 92%, 76%, 64%, and 56%, respectively. Durable responses were observed in patients with high-risk features at baseline (EMD, BMPC ≥60%, or B2M ≥5.5 mg/L) and in patients with high-risk cytogenetics.
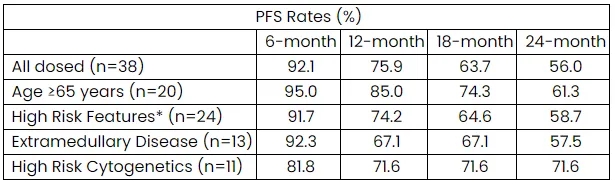
Kaplan-Meier Estimated PFS Rates
Anito-cel is a BCMA-specific CAR-modified T-cell therapy developed by Arcellx, utilizing the company’s novel BCMA-targeting binding domain to treat patients with relapsed or refractory multiple myeloma. Anito-cel is currently in Phase 2 clinical studies and has been granted Fast Track designation, Orphan Drug designation, and Regenerative Medicine Advanced Therapy (RMAT) designation by the U.S. FDA.
As of the October 15, 2023, data cutoff, the dosing regimen of 1.15 billion (+/-10) CAR+ T cells remained well-tolerated. Treatment-emergent adverse events, including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), were manageable with anito-cel. No Grade 3 or higher CRS events were observed, and only one Grade 3 ICANS event (3%) was reported, with no other adverse events of higher grade reported. No on-target/off-tumor toxicities were observed, and no delayed neurotoxicity events or parkinsonism were reported.
Founded in 2014, Arcellx is developing novel, adaptable, and controllable immune cell therapies, including its proprietary synthetic binding scaffold D-Domain, as well as its ddCAR platform and ARC-SparX platform based on D-Domain. Late last year, Arcellx entered into a global strategic collaboration with Kite Pharma to jointly develop and commercialize Arcellx’s lead candidate CART-ddBCMA, with Arcellx receiving a $225 million upfront cash payment and a $100 million equity investment, as well as potential future milestone payments.
Content Source:优尼科尔
