Multiple Myeloma and CAR-T,74% Reduced Risk of Multiple Myeloma Progression,BCMA-Targeted CAR-T Cell Therapy Advanced to Second-Line Treatment
Multiple Myeloma and CAR-T,74% Reduced Risk of Multiple Myeloma Progression,BCMA-Targeted CAR-T Cell Therapy Advanced to Second-Line Treatment
Advancement in Treatment Line – Ciltacabtagene Autoleucel Receives Additional FDA Approval
Ciltacabtagene Autoleucel (Carvykti, cilta-cel), a chimeric antigen receptor T-cell (CAR-T) therapy targeting B-cell maturation antigen (BCMA), has been approved by the FDA on April 6, 2024, for the treatment of relapsed or refractory multiple myeloma (RRMM) in patients who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), and are refractory to lenalidomide. With this new indication for the one-time infusion therapy, Ciltacabtagene Autoleucel becomes the first BCMA-targeted CAR-T therapy globally approved for the second-line treatment of MM!

Figure 1: Ciltacabtagene Autoleucel approved by the FDA for the second-line treatment of RRMM [1]
The Treatment Dilemma in MM: Early Resistance and High Treatment Attrition
Most MM patients relapse after standard treatment, and their condition progressively worsens with each subsequent line of therapy. However, the increasing frequency of early treatment resistance and the high treatment attrition rate in MM (only 13%-35% of patients can receive four or more lines of treatment) have increased the urgent need for new therapies for RRMM patients [2]. Studies have shown that BCMA-targeted CAR-T therapies can induce early, deep, and durable responses in RRMM patients [2]. The approval of Ciltacabtagene Autoleucel for second-line treatment, based on the pivotal CARTITUDE-4 study (NCT04181827), provides detailed and compelling efficacy data.
Figure 2: CAR-T cells binding to the BCMA protein on the surface of myeloma cells [3]
Basis for Ciltacabtagene Autoleucel’s Second-Line Treatment Approval: The CARTITUDE-4 Study
The Phase 3 CARTITUDE-4 trial (NCT04181827) aimed to evaluate the efficacy and safety of Ciltacabtagene Autoleucel compared to standard treatment (PVd* or DPd*) in adult patients with relapsed and lenalidomide-refractory MM who had received 1-3 prior lines of therapy.
*PVd: Pomalidomide, Bortezomib, and Dexamethasone; DPd: Daratumumab, Pomalidomide, and Dexamethasone
The primary endpoint of the CARTITUDE-4 study was progression-free survival (PFS). With a median follow-up of 16 months, the results showed:
1. A 74% reduction in the risk of disease progression or death in the Ciltacabtagene Autoleucel group (HR=0.26; P<0.0001);
2. A significantly higher objective response rate (ORR) in the Ciltacabtagene Autoleucel group (84.6% vs. 67.3%; P<0.0001);
3. A higher 12-month PFS rate in the Ciltacabtagene Autoleucel group compared to the standard treatment group: 75.9% (95% CI: 69.4-81.1) vs. 48.6% (95% CI: 41.5-55.3);
4. The safety profile of Ciltacabtagene Autoleucel was consistent with previous studies: fewer deaths from any cause in the Ciltacabtagene Autoleucel group (39 vs. 46; HR=0.78, 95% CI: 0.5-1.2); 76.1% of patients in the Carvykti group experienced cytokine release syndrome (CRS) (1.1% were Grade 3 or 4, no Grade 5 events), and 4.5% experienced immune effector cell-associated neurotoxicity syndrome (ICANS) (all Grade 1 or 2 events).
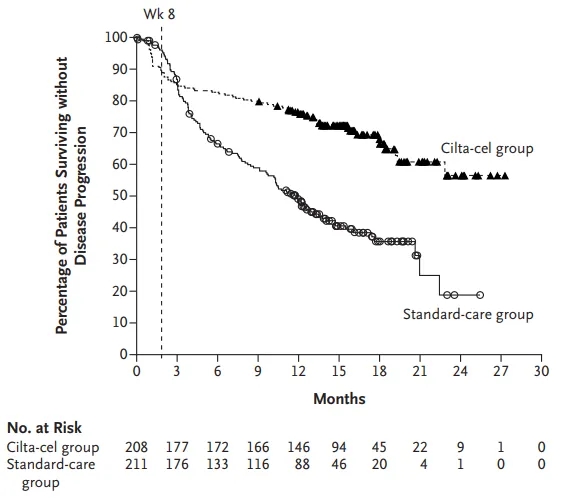
Figure 3: Progression-Free Survival Analysis in the CARTITUDE-4 Study [2]
Summarize
These results indicate that, for lenalidomide-refractory MM patients who have received 1-3 prior lines of therapy, a single infusion of Ciltacabtagene Autoleucel reduces the risk of disease progression or death more effectively than standard treatment. Ciltacabtagene Autoleucel’s approval for second-line treatment provides a more convenient and effective treatment option for patients with first relapse of MM.
The new indication for Ciltacabtagene Autoleucel offers physicians and patients a personalized cell immunotherapy that can be used earlier in the treatment course, potentially bringing hope to more RRMM patients.
Literature Citation
[1]https://investors.legendbiotech.com/news-releases/news-release-details/legend-biotechs-carvyktir-ciltacabtagene-autoleucel-becomes
[2] San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J Med. 2023 Jul 27;389(4):335-347. doi: 10.1056/NEJMoa2303379. Epub 2023 Jun 5. PMID: 37272512.
[3] https://www.cancer.gov/news-events/cancer-currents-blog/2022/fda-carvykti-multiple-myeloma
Content Source:捷信医药科技
