GPRC5D Targeted CAR-T Cells for Myeloma
GPRC5D Targeted CAR-T Cells for Myeloma
In addition to serving as an antibody target, GPRC5D is a promising CAR target. Introduction to a CART targeting GPRC5D.

Main Content
Early clinical results of chimeric antigen receptor (CAR) T-cell therapy targeting B-cell maturation antigen (BCMA) for the treatment of multiple myeloma (MM) appear promising, but relapses associated with low to negative BCMA expression on MM cells have been reported, necessitating the discovery of additional novel targets.
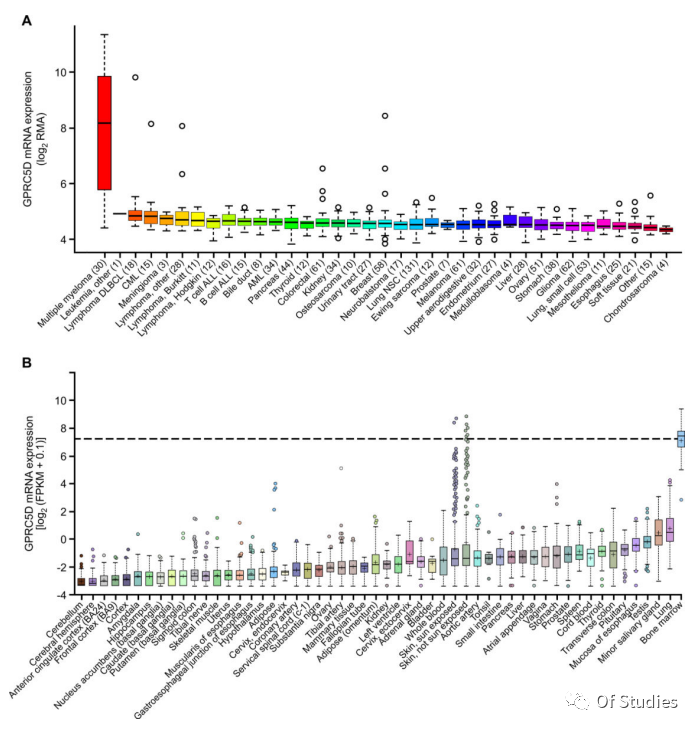
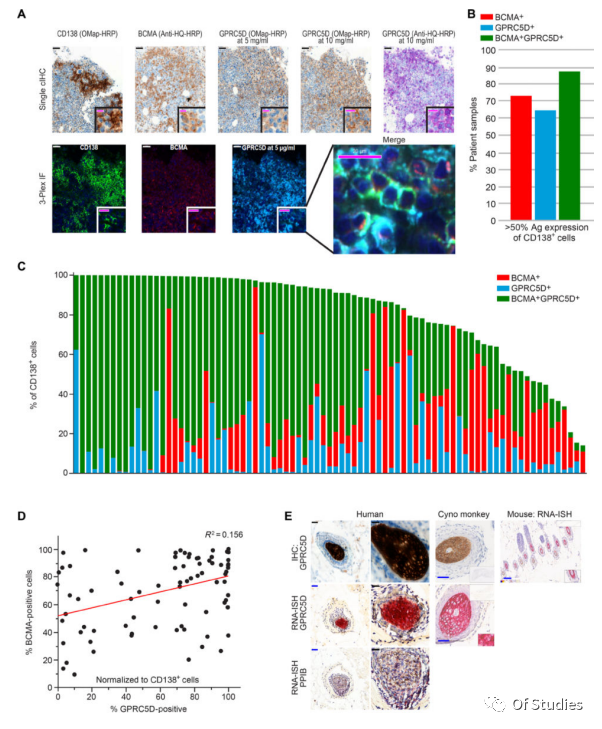
The orphan G protein-coupled receptor, family C, group 5, member D (GPRC5D), typically expressed only in hair follicles, was previously identified as being expressed at the mRNA level in bone marrow aspirates from MM patients, but confirmation of protein expression has remained elusive. Using quantitative immunofluorescence, the authors determined that GPRC5D protein is expressed on CD138+ MM cells from primary bone marrow samples, with a distribution similar to but independent of BCMA. Panning of a human B cell–derived phage display library identified seven GPRC5D-specific single-chain variable fragments (scFvs).
CAR T cells containing the GPRC5D-targeting scFv clone 109 eliminated MM tumor cells and achieved long-term survival, including in a BCMA antigen-escape model. GPRC5D (scFv clone 109) is specific for GPRC5D and did not induce alopecia or other signs of GPRC5D-mediated toxicity in vivo. Thus, irrespective of prior BCMA-targeted therapy, GPRC5D (scFv clone 109) CAR T-cell therapy shows potential for treating advanced MM.
Key Findings
1. High expression of GPRC5D mRNA in MM cells and variable expression in skin
2. Expression of GPRC5D protein in primary MM cells and hair follicles
3. Development of GPRC5D-targeted CARs – Exploration of CAR structures
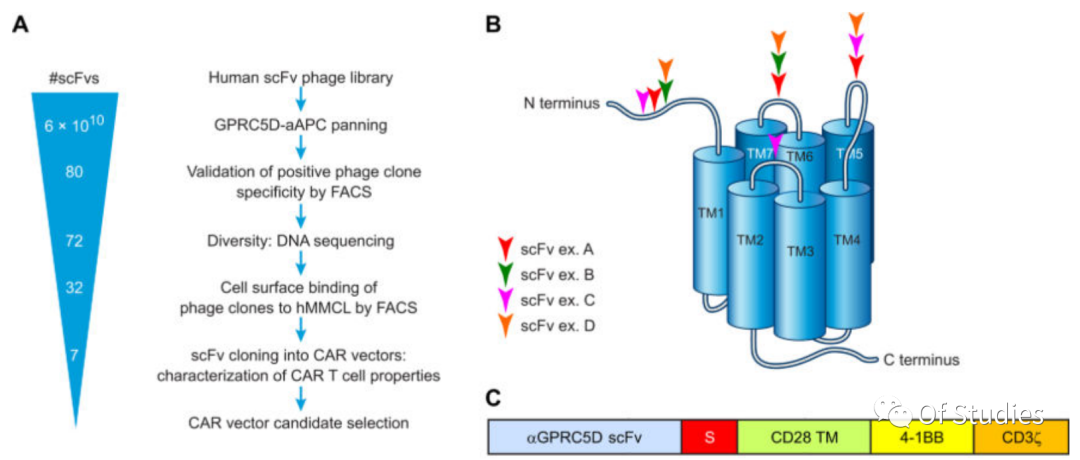
GPRC5D here is a fully human antibody, screened from a human scFv phage library.
To select and identify GPRC5D-specific single-chain variable fragments (scFvs), NIH-3T3 fibroblasts were stably transduced with human GPRC5D cDNA by retroviral transduction to generate stable artificial antigen-presenting cells (hGPRC5D-aAPCs). These cells were confirmed for GPRC5D expression by flow cytometry, and subclones with high antigen expression were expanded. The hGPRC5D-aAPCs were used to screen a human B cell–derived scFv phage display library for scFvs (screening and validation strategy). Ultimately, 32 distinct clones were identified, encompassing 5 and 3 subfamilies for the light chain and heavy chain complementarity-determining regions (CDRs), respectively, with HCDR3 lengths ranging from 6 to 23 amino acids. The top seven clones with highest specificity for binding to the human MM cell lines MM.1S and NCI-H929 but not to GPRC5D-negative cell lines (derived from other hematologic malignancies) were selected for development into CAR constructs. Epitope mapping of a subset of these scFv clones revealed distinct epitope binding.
To select an scFv for clinical development as a CAR, the authors designed CARs targeting GRPC5D with the 7 scFvs, incorporating various structural forms:
– the variable heavy chain/variable light chain (VH/VL) or VL/VH orientation of each scFv
– each with one of three IgG4/IgG2–derived spacer domains of varying lengths [short, hinge only, 12 amino acids; medium, hinge-CH3, 119 amino acids; or long, hinge-CH2-CH3, 228 amino acids, with CH2 modifications to limit Fc receptor binding as previously described]
– for a total of 42 CAR constructs.
All CARs designed in this initial evaluation incorporated a CD28 transmembrane domain and 4-1BB and CD3ζ signaling domains (Fig. 3C).
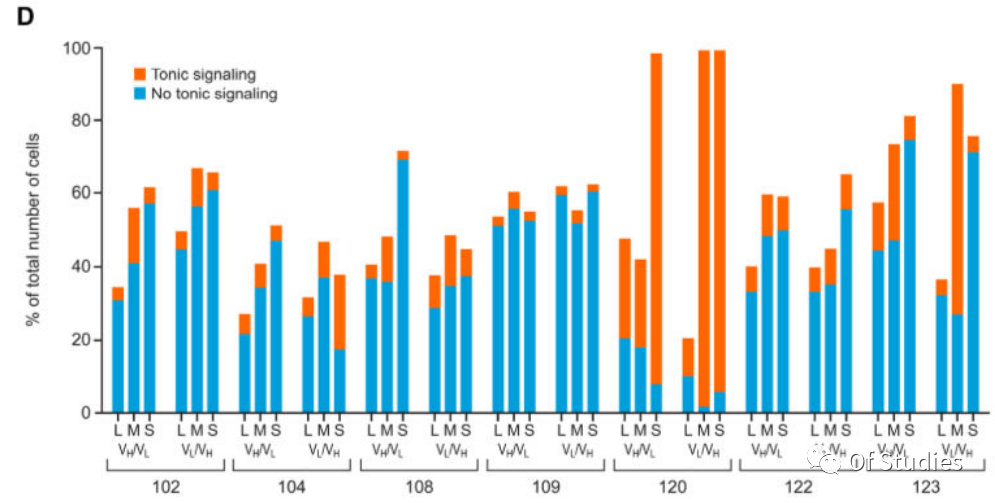
Due to antigen-independent (tonic) signaling potentially impairing the overall efficacy of CAR T-cell therapy, CARs that conveyed limited tonic signaling were first screened. A reporter Jurkat T-cell line was generated that specifically expresses red fluorescent protein (RFP) downstream of CD3ζ signaling. The Nur77-RFP Jurkat T-cell line was stably transduced with bicistronic constructs containing GFP and one of the 42 CAR constructs, and tonic signaling was determined as the percentage of GFP (CAR-transduced) cells that were also RFP positive. The readout varied widely among CAR constructs, with constructs incorporating the GPRC5D-targeting scFv 109 [GPRC5D(109)] consistently showing the least tonic signaling as indicated by this assay (Fig. 3D). Certain CAR constructs associated with the highest tonic signaling in cells expressing these CARs also suppressed growth of the Jurkat reporter cell line and were excluded from further evaluation (120 VH/VL and VL/VH with the short spacer and 123 VL/VH with the medium spacer).
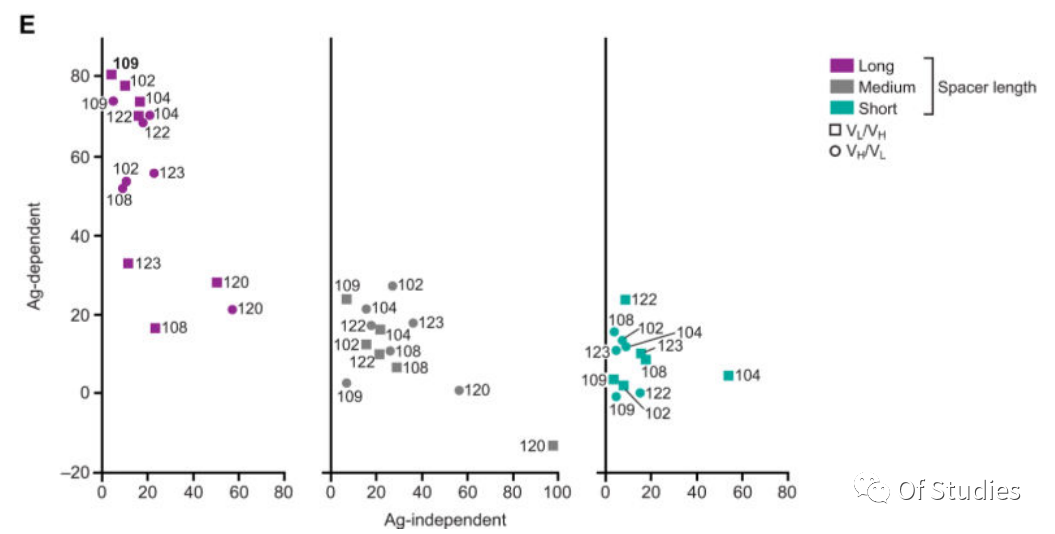
The CAR/GFP-modified Jurkat Nur77-RFP reporter cells were cocultured 1:2 with the MM.1S myeloma cells that endogenously express GPRC5D, and RFP was measured at 20 hours. The readout indicated that incorporation of the long spacer increased antigen-mediated signaling through the CAR but did not enhance antigen-independent (tonic) signaling, with the GPRC5D(109) CAR in the VL/VH orientation with a long spacer being most sensitive to antigen exposure and displaying the lowest tonic signaling (Fig. 3E).
4. Specificity of scFv clone 109 for GPRC5D
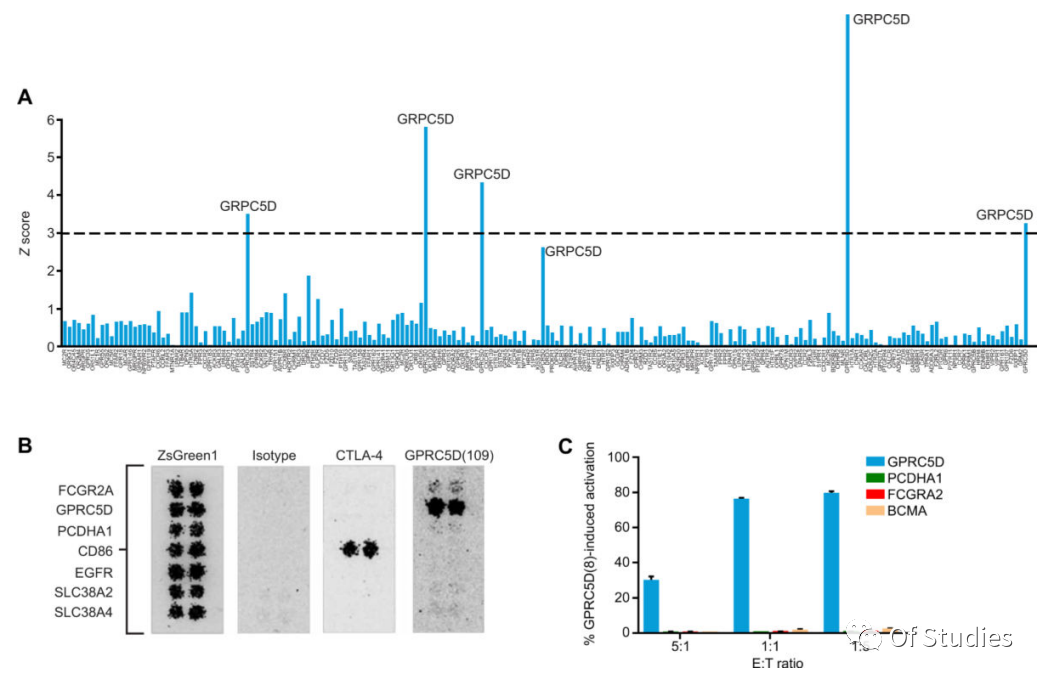
To assess the potential for off-target binding of the anti-GPRC5D clone 109, its specificity was measured across G protein–coupled receptors (GPCRs). HEK293 cells were transiently transfected with the anti-GPRC5D scFv clone 109, including the long spacer, in a cell surface display vector also encoding cytoplasmic mCherry; concurrently, the cDNA for each human GPCR was transiently expressed in a vector encoding cytoplasmic GFP, with 202 passing quality control for >25% transfection and screened for off-target binding. Using an automated flow cytometry assay to detect cell–cell interactions, the scFv clone 109 as a cell surface chimera was determined to bind only GPRC5D.
Specificity of clone 109 for GPRC5D within cell surface proteins was further confirmed using an scFv-Fc IHC assay, in which single HEK293 cell clusters (each expressing one of 4,417 human membrane proteins) were grown in microarray spots and treated with either anti-GPRC5D clone 109 scFv-mIgG2a Fc antibody or mIgG2a Fc isotype control. After treatment with fluorescently labeled secondary antibody, the cell microarrays were evaluated for binding by automated fluorescence microscopy. The antibody containing clone 109 bound strongly to GPRC5D and initially indicated two additional proteins, protocadherin alpha 1 (PCDHA1) and Fcγ receptor 2A (CD32a; FCGR2A), a protein with known Fc interaction potential. A small-scale secondary assay including only these proteins indicated possible binding (Fig. 5B).
Nonetheless, when K562 cells expressing these proteins were individually cocultured with Jurkat Nur77-RFP reporter gene cells expressing the GPRC5D(109) CAR, neither coculture resulted in activation as measured by RFP signal (Fig. 5C), confirming that these potential off-target interactions were nonspecific and did not stimulate GPRC5D(109) CAR signaling in the presence of these proteins. Furthermore, activation of the GPRC5D(109) CAR-expressing Jurkat Nur77-RFP reporter cells mediated by coculture with the OPM2 MM cells was abrogated upon coculture with OPM2 cells in which GPRC5D was knocked out using CRISPR-Cas9 (Fig. S8). In summary, these results indicate that the scFv clone 109 specifically recognizes GPRC5D.
References
Smith EL, Harrington K, Staehr M, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485):eaau7746. doi:10.1126/scitranslmed.aau7746.
Content Source:Of Studies
