Carvykti Target is BCMA, Brief Introduction of BCMA Target
Carvykti Target is BCMA, Brief Introduction of BCMA Target
Carvykti Target is BCMA
BCMA (B-Cell Maturation Antigen) is a specific target for multiple myeloma, and therapies targeting BCMA are considered an effective approach to improve the quality of life for patients with multiple myeloma. In the first half of 2022, Carvykti, a BCMA CAR-T product co-developed by Legend Biotech and Johnson & Johnson, received marketing approvals from the FDA and EMA based on its outstanding clinical trial data for the treatment of relapsed or refractory multiple myeloma in patients who have received at least five prior lines of therapy (5L+) or at least four prior lines of therapy (4L+), respectively. Carvykti is currently regarded as the best-in-class product.
Carvykti employs a second-generation CAR-T structure with a murine-derived scFv and the 4-1BB co-stimulatory domain. It utilizes a lentiviral vector and incorporates two single-domain antibodies targeting BCMA in the extracellular design, conferring stronger binding affinity. The FDA approval of Carvykti was primarily based on the clinical trial data from CARTITUDE-1, a Phase Ib/II study conducted in the United States and Japan. CARTIFAN-1, a registrational Phase II study of Carvykti in China, is also ongoing.
The CARTITUDE-1 study enrolled 97 patients who had received a median of six prior lines of therapy, including 29 patients in Phase Ib and 68 patients in Phase II. Legend Biotech presented updated data from the CARTITUDE-1 trial at the ASCO 2022 meeting, showing an overall response rate (ORR) of 98% with a median follow-up of 28 months. Earlier follow-up data indicated that 83% of patients achieved stringent complete response (sCR) at the median follow-up of 28 months, with a 2-year progression-free survival (PFS) rate of 55% and an overall survival (OS) rate of 70%. Furthermore, among 61 patients evaluable for minimal residual disease (MRD), 92% achieved MRD negativity, with 68% sustaining MRD negativity for over 6 months and 55% sustaining MRD negativity for over 12 months. In terms of safety, 5.1% of patients experienced grade 3 or higher cytokine release syndrome (CRS), and 12.3% experienced neurotoxicity, indicating a favorable safety profile.
BCMA: An Overview
BCMA, also known as B-Cell Maturation Antigen, TNFRSF17, BCM, or CD269, is a member of the tumor necrosis factor receptor superfamily and was first discovered in the early 1990s. It is a single-pass transmembrane glycoprotein consisting of 184 amino acids and plays a crucial role in B-cell maturation and differentiation into plasma cells (PCs).
1. BCMA Gene and Protein Structure
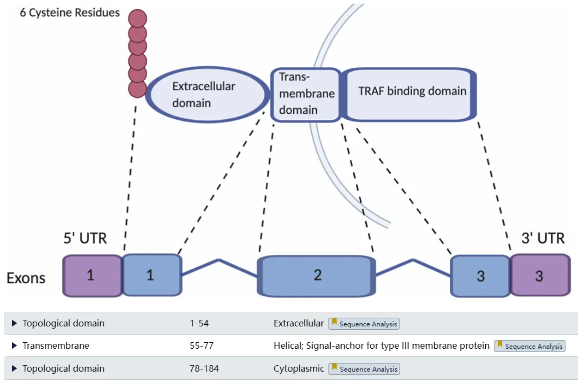
BCMA comprises three main structural domains: an extracellular domain (EC, Aa1-54, with disulfide bonds between positions 8-21, 24-37, and 28-41), a transmembrane domain (TM, Aa 55-77), and an intracellular domain (Aa 78-184).
2. BCMA Expression
In Normal Tissues:
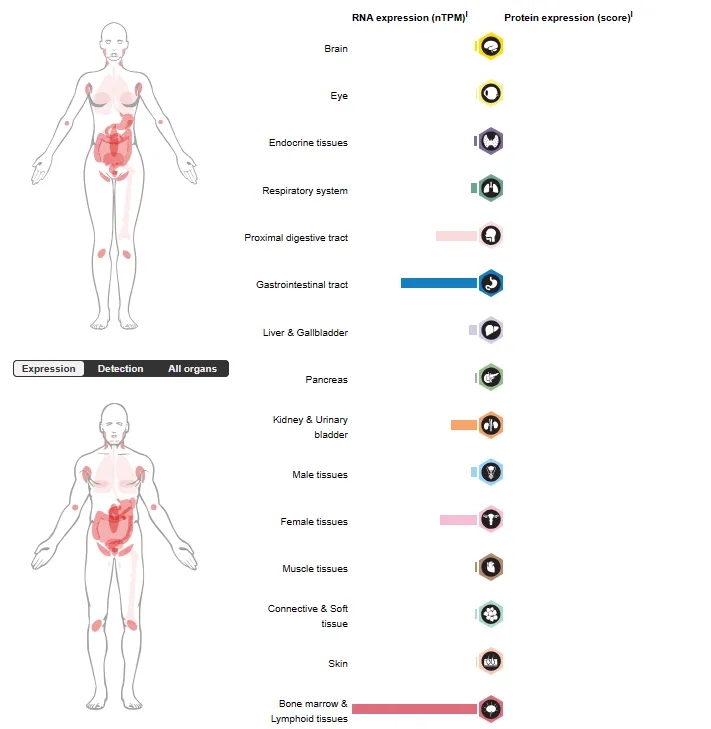
Source: Tissue expression of TNFRSF17 – Summary – The Human Protein Atlas
In normal human tissues, BCMA protein and mRNA are almost exclusively found on plasma cells, and it is selectively overexpressed during the malignant transformation of plasma cells.
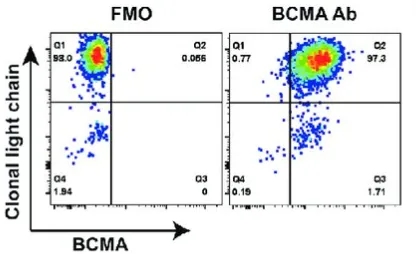
In Malignant Plasma Cells:
BCMA is highly expressed in malignant plasma cells but not expressed in normal tissue cells (except plasma cells and some mature B cells) or CD34+ stem/progenitor cells, making it a promising targeting site for multiple myeloma (MM). In pathological conditions, BCMA is expressed in almost all MM tumor cell lines (80%-100%).
3. BCMA-Targeted Therapies
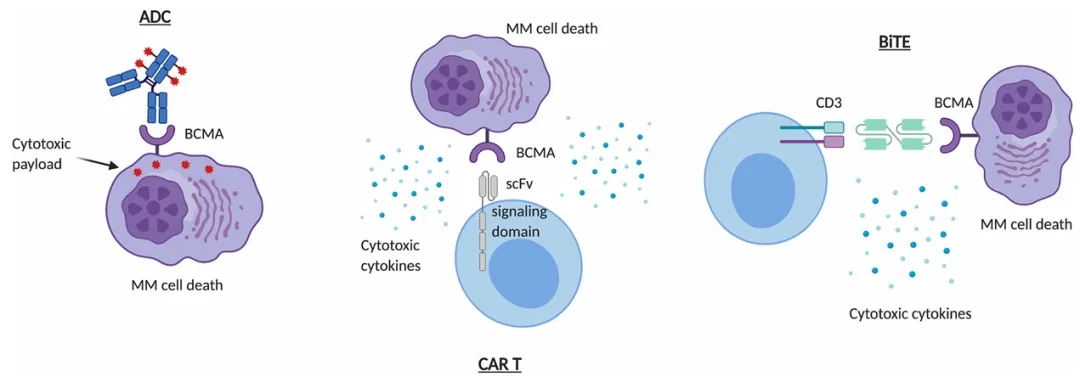
Among BCMA-targeted therapies, antibody-drug conjugates (ADCs) and CAR-T cells have made significant progress. Legend Biotech’s BCMA-CAR-T (ciltacabtagene autoleucel) has been approved in the United States, and GlaxoSmithKline’s BCMA-ADC (Blenrep) received FDA approval in 2020.
4. BCMA Signaling Pathway
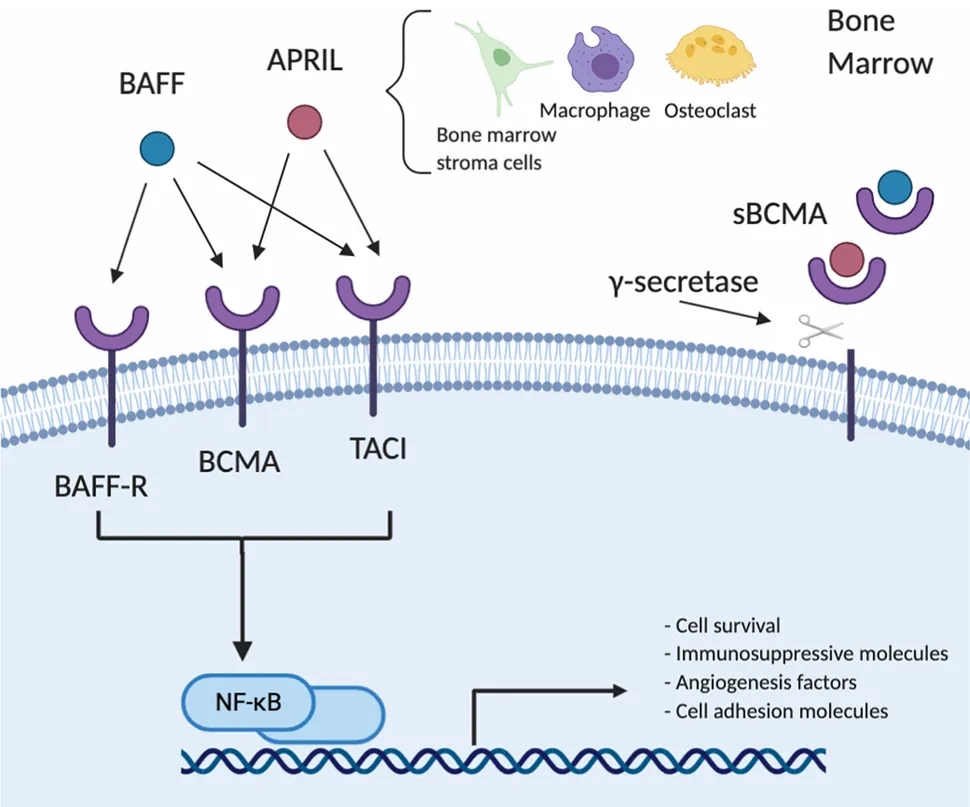
BCMA primarily activates the NFκB, AKT, phosphoinositide 3-kinase (PI3K), STAT3, and MAPK intracellular signaling cascades, promoting tumor cell growth and survival.
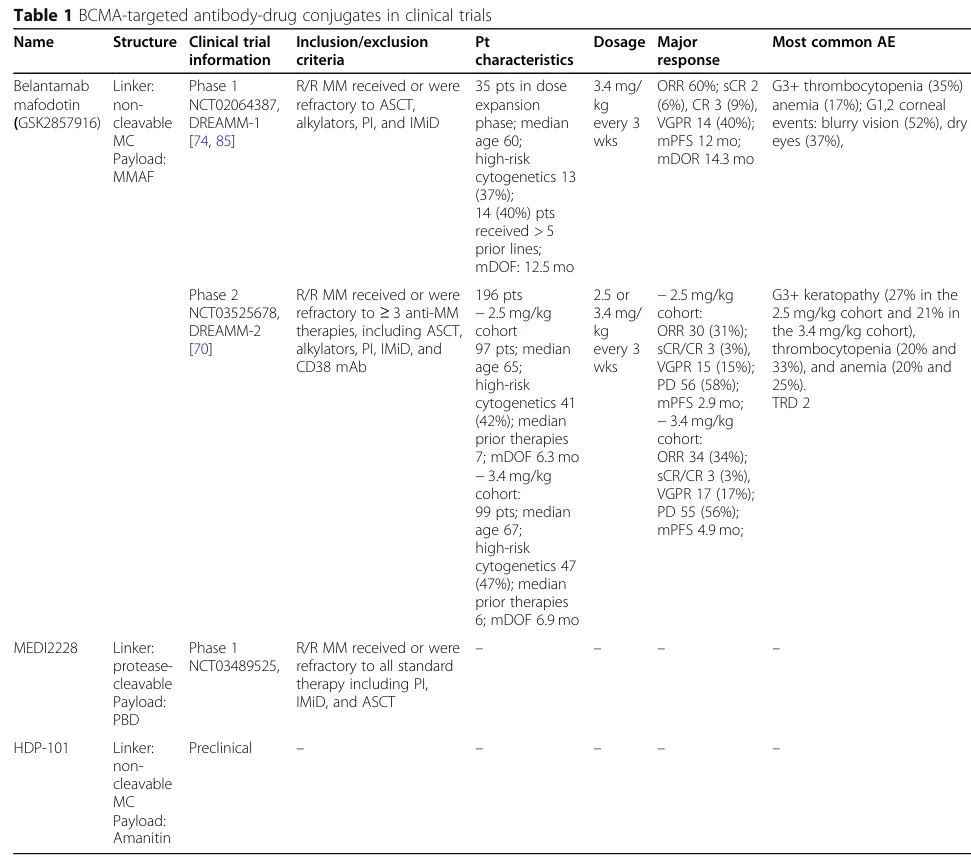
5. Clinical Trials of BCMA-Targeted ADCs
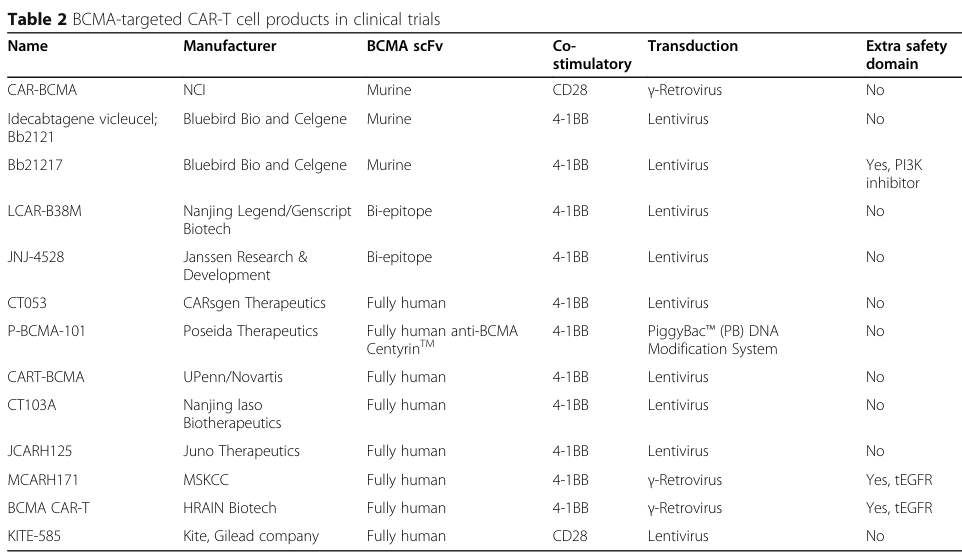
6. Clinical Trials of BCMA CAR-T Therapies
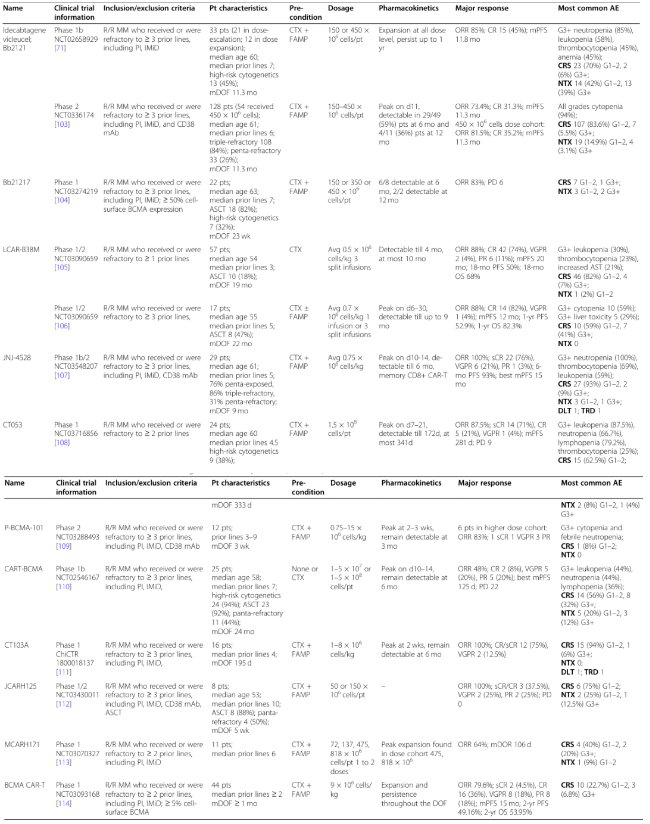
7. Clinical Trial Designs and Results of BCMA CAR-T Therapies
8. BCMA-Targeted Bispecific Antibodies
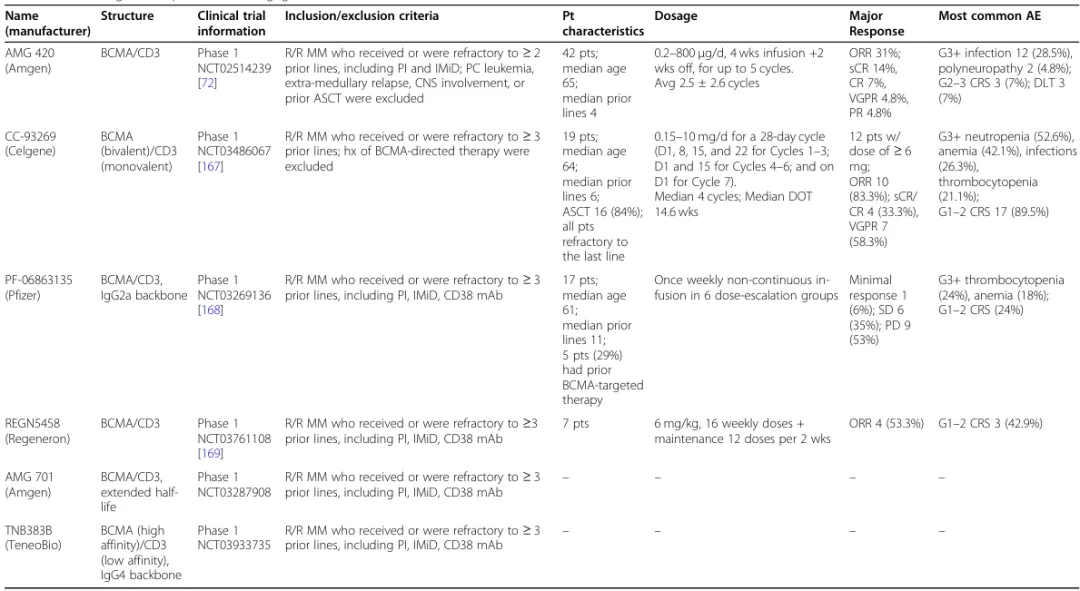
BCMA is a specific biomarker for normal and malignant plasma cells. Various BCMA-targeted drugs, including ADCs, CAR-T cells, and BiTEs, are currently undergoing active clinical development.
Content Source:CPHI制药在线+Of Studies
