Zevorcabtagene Autoleucel (CT053): China’s Second BCMA-Targeted CAR-T Cell Therapy for Relapsed/Refractory Multiple Myeloma
Zevorcabtagene Autoleucel (CT053): China’s Second BCMA-Targeted CAR-T Cell Therapy for Relapsed/Refractory Multiple Myeloma
On March 1, 2024, blood tumor patients welcomed another encouraging piece of news. China’s National Medical Products Administration (NMPA) officially issued a notification approving the New Drug Application (NDA) for Zevorcabtagene Autoleucel (CT053) injection, developed by Keymed Biosciences, for the treatment of relapsed or refractory multiple myeloma (approval date: February 23, 2024).
Notably, Zevorcabtagene Autoleucel is the fifth CAR-T cell product and the second BCMA-targeted CAR-T product approved for marketing in China.
About Zevorcabtagene Autoleucel
Zevorcabtagene Autoleucel (zevor-cel, trade name Saikeize®, product code CT053) is an autologous BCMA-targeted CAR-T cell product generated through lentiviral transduction of T cells. The lentivirus-encoded CAR comprises a fully human BCMA-specific single-chain variable fragment (scFv), CD8α transmembrane domain, human CD8α hinge domain, CD3ζ activation domain, and 4-1BB costimulatory domain, exhibiting high binding affinity and stability.
This product is primarily indicated for the treatment of adult patients with relapsed or refractory multiple myeloma (R/R MM) who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, and have experienced disease progression.
Zevorcabtagene Autoleucel received Regenerative Medicine Advanced Therapy (RMAT) and Orphan Drug designations from the U.S. Food and Drug Administration (FDA) in 2019. It also received the PRIME (PRIority MEdicines) designation from the European Medicines Agency (EMA) in the same year and was granted Breakthrough Therapy designation by the NMPA in 2020.
Zevorcabtagene Autoleucel: A Game-Changer for Multiple Myeloma, ORR Reaches 100%
The NMPA’s approval for Zevorcabtagene Autoleucel to be marketed is primarily based on the results of an open-label, multicenter Phase I/II clinical trial (LUMMICAR STUDY 1, NCT03975907) conducted in China.
Starting from July 23, 2019, a total of 14 patients with relapsed or refractory multiple myeloma (R/R MM) were enrolled. The median age was 54 years, with an ECOG performance status of 0 or 1. All patients had received at least three prior lines of therapy, including at least one immunomodulatory agent and one proteasome inhibitor. After lymphodepleting chemotherapy 5 to 7 days prior, these patients received a single infusion of Zevorcabtagene Autoleucel (zevor-cel). With a median follow-up of 37.7 months as of July 17, 2023, the results were as follows:
1. Overall Response Rate (ORR): The ORR reached 100% (14/14), with 11 patients (78.6%) achieving complete response (CR) or stringent complete response (sCR), and 3 patients (21.4%) achieving partial response (PR).
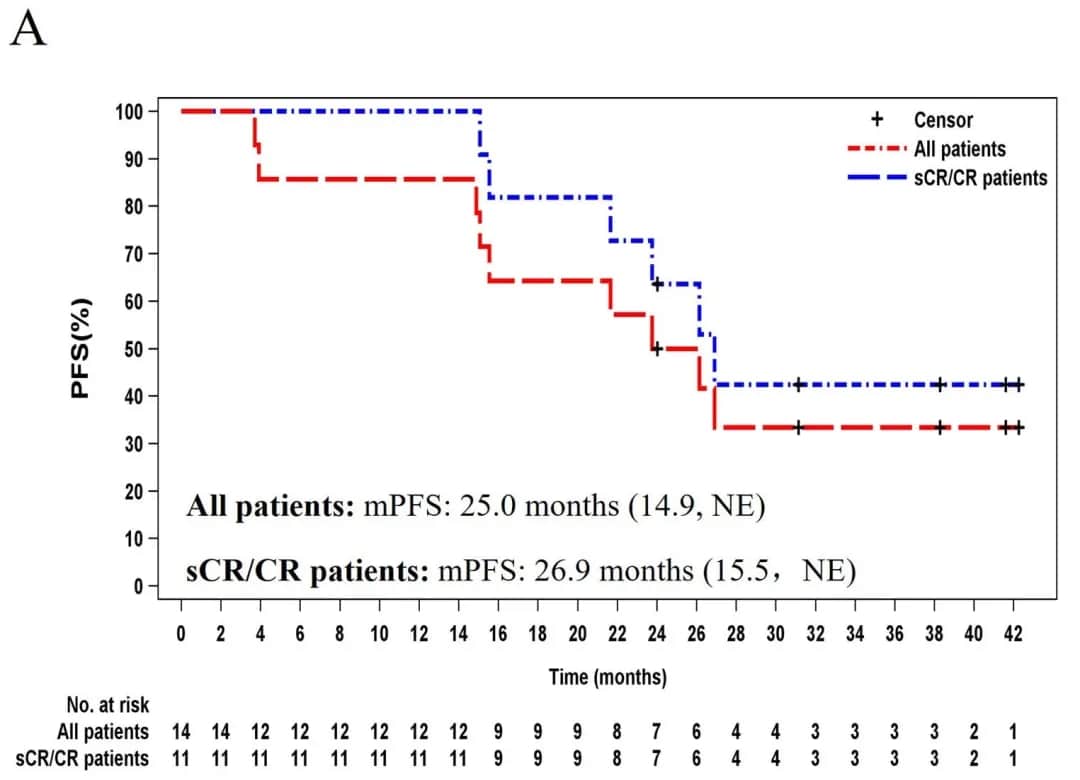
2. Progression-Free Survival (PFS): The median PFS for all patients was 25.0 months (14.9, not evaluable [NE]). For sCR/CR patients, the median PFS was 26.9 months (15.5, NE) (Figure 1A).
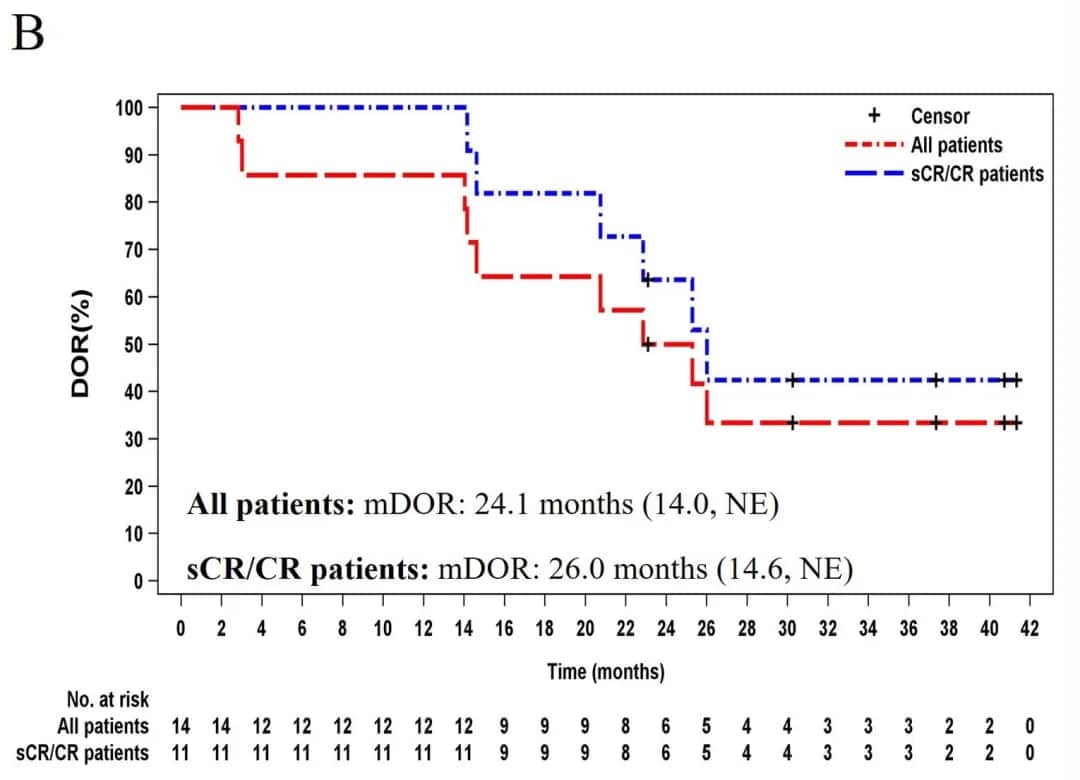
3. Duration of Response (DOR): The median DOR for all patients was 24.1 months (14.0, NE). For sCR/CR patients, the median DOR was 26.0 months (14.6, NE) (Figure 1B).
In summary, heavily pretreated patients with relapsed or refractory multiple myeloma (R/R MM) who received a single infusion of zevor-cel demonstrated encouraging safety and maintained deep and durable responses after approximately 3 years of follow-up.
