FDA Approval CAR-T Multiple Myeloma
FDA Approval CAR-T Multiple Myeloma
CAR-T cell therapy (Chimeric Antigen Receptor T-cell therapy) is a form of cellular immunotherapy that involves genetically reprogramming a patient’s own T cells by inserting a DNA segment encoding a CAR, to induce an anti-tumor response.
Currently, the B-cell maturation antigen (BCMA) is a widely used target for CAR-T cell therapy in treating relapsed or refractory multiple myeloma, bringing new hope to patients.
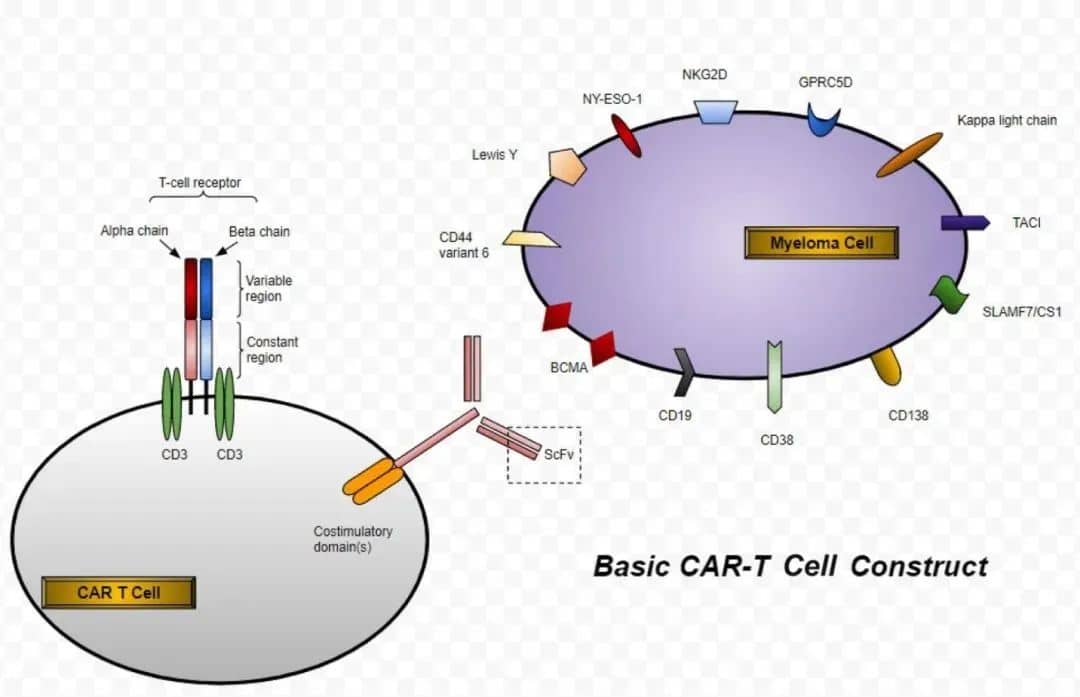
As of April 2024, the FDA has approved two CAR-T cell therapies for the treatment of multiple myeloma, Abecma (Idecabtagene vicleucel) and Carvykti (Ciltacabtagene Autoleucel, cilta-cel). Here is a brief introduction to these two CAR-T cell therapies.
Abecma (idecabtagene vicleucel)
Product Name: Abecma
Generic Name: idecabtagene vicleucel
Developer: Bristol-Myers Squibb
Indication: For the treatment of relapsed or refractory multiple myeloma after three or more lines of therapy
Abecma (idecabtagene vicleucel, ide-cel) is the first FDA-approved CAR-T cell immunotherapy targeting B-cell maturation antigen (BCMA), a crucial B-cell biomarker widely expressed on the surface of multiple myeloma cells. Abecma can recognize and bind to the BCMA protein on myeloma cells, leading to the death of BCMA-expressing cancer cells.
Initial Approval:
On March 26, 2021, Bristol-Myers Squibb and bluebird bio jointly announced that the FDA had approved Abecma (idecabtagene vicleucel, ide-cel) for the treatment of relapsed or refractory multiple myeloma in adult patients after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
The FDA approval was based on data from the pivotal KarMMa Phase 2 study, which enrolled 127 patients with relapsed/refractory multiple myeloma who had previously received at least three prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody. Of the 100 efficacy-evaluable patients, 88% had received four or more prior lines of therapy, and 85% were triple-class refractory.
Second Approval:
On April 9, 2024, the U.S. Food and Drug Administration (FDA) approved Abecma (idecabtagene vicleucel) as a personalized CAR-T cell therapy for the treatment of relapsed or refractory multiple myeloma after three or more lines of therapy.
The approval is for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior therapies, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
The approval was based on data from the Phase 3 KarMMa-3 trial involving 254 patients. At a median follow-up of 15.9 months, the primary endpoint of progression-free survival with Abecma was more than triple that of the standard regimen, with a median progression-free survival of 13.3 months versus 4.4 months (hazard ratio 0.49). The overall response rate was also significantly improved with Abecma, with 71% of patients achieving a response compared to 42% in the standard therapy group, and 39% achieving a complete or stringent complete response versus 5% in the standard therapy group. Responses to Abecma were durable, with a median response duration of 14.8 months (or 20 months for patients achieving a complete response or better).
Carvykti (Ciltacabtagene Autoleucel, cilta-cel)
Product Name: CARVYKTI
Generic Name: Ciltacabtagene Autoleucel
Developer: Legend Biotech USA
Indication: For the treatment of adult patients with relapsed or refractory multiple myeloma, who have received at least one prior line of therapy, including a proteasome inhibitor and an immunomodulatory agent, and are refractory to Lenalidomide.
Carvykti is a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell (CAR-T) therapy, which uses a genetically modified version of a patient’s own T cells with a CAR to recognize and eliminate BCMA-expressing cells.
Initial Approval:
On February 28, 2022, Legend Biotech’s BCMA-targeted CAR-T therapy, Carvykti (ciltacabtagene autoleucel), was approved in the United States for the treatment of relapsed or refractory multiple myeloma (R/R MM) patients.
The approval was based on data from the pivotal CARTITUDE-1 study, an open-label, multicenter, Phase 1b/2 study in adult patients with relapsed or refractory multiple myeloma who had previously received multiple prior lines of therapy, including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and anti-CD38 antibodies.
According to the latest data presented at the 63rd American Society of Hematology (ASH) Annual Meeting in 2021, with nearly two years of follow-up in 97 patients with relapsed or refractory multiple myeloma, the overall response rate was 98%, the stringent complete response rate was 83%, and the two-year progression-free survival and overall survival rates were 61% and 74%, respectively.
The median time to first response was one month, the median time to best response was 2.6 months, and the median time to complete response or better was 2.9 months.
Second Approval:
On April 5, 2024, Johnson & Johnson announced that the U.S. Food and Drug Administration (FDA) has approved CARVYKTI (ciltacabtagene autoleucel; cilta-cel) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least one prior line of therapy, including a proteasome inhibitor and an immunomodulatory agent, and are refractory to Lenalidomide. With this approval, CARVYKTI becomes the first and only FDA-approved B-cell maturation antigen (BCMA)-directed therapy for the treatment of multiple myeloma.
The FDA approval was based on the positive results from the CARTITUDE-4 Phase 3 study, which showed that early use of CARVYKTI reduced the risk of disease progression or death by 59% compared to standard therapy (Pomalidomide, Bortezomib, and Dexamethasone (PVd) or Daratumumab, Pomalidomide, and Dexamethasone (DPd)) in adult patients with relapsed and Lenalidomide-refractory multiple myeloma who had received one to three prior lines of therapy. The study was presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting and published in the New England Journal of Medicine, also reporting key secondary endpoints such as overall response (OR) and overall survival (OS).
The above summarizes the two FDA-approved CAR-T cell therapies for the treatment of multiple myeloma and their respective indications. Regarding pricing, Abecma costs $438,000 per dose, and Carvykti costs $465,000 per dose in the United States, which is unaffordable for most families. Additionally, China has two CAR-T cell therapies approved for multiple myeloma, which are significantly more affordable at around 1.15 million Chinese yuan. If you are interested, you can inquire about the Chinese CAR-T cell therapies as well.
