High Risk Multiple Myeloma Solution and Chinese Expert Consensus
**High Risk Multiple Myeloma Solution and Chinese Expert Consensus**
Multiple Myeloma
High risk multiple myeloma (HRMM) refers to patients with multiple myeloma whose overall survival is less than 2 to 3 years under current standard treatments.
In 2024, the Chinese Society of Clinical Oncology’s Multiple Myeloma Expert Committee and the Chinese Anti-Cancer Association’s Hematologic Oncology Committee, organized by relevant experts, developed the “Chinese Expert Consensus on the Diagnosis and Treatment of High Risk Multiple Myeloma (2024 Edition),” which was officially published in the *Chinese Journal of Hematology* in May 2024. This consensus defines HRMM, outlines high-risk factors and risk stratification systems, and provides key treatment recommendations for HRMM, aiming to improve the quality of life and prognosis for HRMM patients in China.
**Definition of HRMM**
There is currently no precise definition of HRMM. Referencing the International Myeloma Working Group (IMWG)’s definition, the Chinese Expert Committee considers HRMM patients as those with an overall survival (OS) of less than 3 years after receiving autologous hematopoietic stem cell transplantation (auto-HSCT) or less than 2 years if they have not received auto-HSCT. Patients with OS of less than 2 years after receiving auto-HSCT are classified as ultra-high risk multiple myeloma (UHRMM) patients.
**Prognostic Factors of HRMM**
The biological characteristics of MM tumor cells and treatment response are key determinants in identifying HRMM.
**Static Prognostic Factors of MM**
-
**Genetic High-Risk Factors:**
In the context of genetic high-risk factors, cytogenetic abnormalities are core indicators in the MM risk stratification system, but there is still some debate over the definition of high-risk cytogenetic abnormalities (HRCAs). The “National Comprehensive Cancer Network (NCCN) Guidelines (2024.v1)” indicate that the presence of multiple HRCAs correlates with a poorer prognosis. Fluorescence in situ hybridization (FISH) is currently the main genetic testing technique for MM, and next-generation sequencing can be performed if conditions allow (TP53 mutations have a significant impact on prognosis, while the effects of KRAS, NRAS, DIS3, BRAF, and FAM46C are less clear). All these genetic tests require enrichment and selection of plasma cells.
-
**Non-Genetic High-Risk Factors:**
Confirmed non-genetic prognostic factors include International Staging System (ISS) stage III, extramedullary disease excluding bone lesions, circulating plasma cells, high plasma cell proliferation index, elevated lactate dehydrogenase (LDH), frailty, renal insufficiency, and thrombocytopenia.
**Dynamic Prognostic Factors of MM**
-
**Duration of Initial Treatment Response:**
The duration of response to initial treatment is a crucial dynamic prognostic factor for MM. Patients who received auto-HSCT followed by maintenance therapy and experienced relapse/progression within less than 2 years are classified as HRMM; for those who did not receive auto-HSCT, relapse within less than 18 months after starting treatment also indicates HRMM. Functional high risk refers to MM patients without known genetic high-risk factors at diagnosis who experience early progression within 18 months after the start of treatment.
-
**Depth of Initial Treatment Response:**
The depth of response to initial treatment is another important dynamic prognostic factor for MM. Patients who achieve negativity for minimal residual disease (MRD) in both bone marrow and imaging studies have the best survival outcomes. Achieving MRD negativity can partially overcome the adverse prognosis associated with high-risk cytogenetics. Continuous dynamic MRD monitoring has greater clinical value than a single MRD result, as sustained MRD negativity for more than 12 months can translate into long-term survival.
The Expert Committee considers newly diagnosed MM to be classified as HRMM if any of the following criteria are met:
-
R-ISS stage III, extramedullary disease excluding bone lesions, presence of circulating plasma cells (plasma cell leukemia is defined as ≥5% plasma cells in peripheral blood), presence of one or more HRCAs [t(4;14), t(14;16), t(14;20), del(17/17p), 1q21 gain/amplification, del(1p32), TP53 mutation], although 1q21 gain alone does not define HRMM;
-
MM patients who have received auto-HSCT followed by maintenance therapy and experience relapse within less than 2 years from the start of treatment;
-
Patients who have not received auto-HSCT and experience relapse within less than 18 months from the start of treatment;
-
Functional high risk;
-
Extramedullary relapse/secondary plasma cell leukemia;
-
New occurrence of 1q21 gain/amplification and/or del(17/17p)/TP53 mutation at relapse.
**Treatment of HRMM**
**Principles of Treatment for Newly Diagnosed HRMM**
The standard treatment for HRMM has not yet been established. The overall treatment strategy includes:
-
Utilizing combination therapies with drugs that have different mechanisms of action;
-
Aiming to eradicate all tumor clones, with the goal of achieving and maintaining MRD negativity both inside and outside the bone marrow;
-
Implementing a treatment strategy that adjusts based on the effectiveness of the therapy;
-
Acknowledging that current treatment outcomes for HRMM are still unsatisfactory, and encouraging the exploration of experimental therapies.
**Treatment for Newly Diagnosed HRMM Suitable for Transplantation**
-
**Induction Therapy Before Transplantation:**
For HRMM, induction therapy with the RVd regimen as a bridge to auto-HSCT has not met expectations in terms of depth of response and long-term prognosis, and achieving MRD negativity is more challenging compared to standard-risk patients. Some studies with novel drug-modified regimens have shown that patients with one HRCA receiving the KRd regimen (carfilzomib, lenalidomide, and dexamethasone) sequentially followed by auto-HSCT achieved similar MRD negativity rates and progression-free survival (PFS) as standard-risk patients, with no statistically significant difference. Meta-analyses indicate that incorporating a CD38 monoclonal antibody as part of the treatment backbone in early-line therapy provides clinical benefits for patients with HRCAs. For UHRMM patients, more intensive treatment regimens, such as the Dara-VRdC regimen (OPTIMUM/MUKnine study) and the Dara+KTD-PACE regimen (TT7 study), can be considered.
-
**Auto-HSCT:**
Tandem transplantation involves performing a planned second auto-HSCT within 3–6 months after the first. It is recommended that HRMM patients collect sufficient hematopoietic stem cells for two auto-HSCTs during the first mobilization. Regardless of the response achieved after the first transplant, it is advised to perform the tandem transplant within six months. The conditioning regimen for both transplants typically includes high-dose melphalan.
-
**Consolidation Therapy:**
If tandem transplantation is not performed, the original induction regimen can be continued for consolidation therapy for an additional 2–4 cycles.
-
**Maintenance Therapy:**
For HRMM patients, maintenance therapy should consider a combination of proteasome inhibitors, immunomodulators, and CD38 monoclonal antibodies, in either dual or triple drug regimens. It is recommended to continue maintenance therapy until disease progression or intolerance.
-
**Allo-HSCT:**
The long-term efficacy of allo-HSCT remains debatable and should only be considered within the context of clinical trials and for select high-risk patients.
#HRMM #AutoHSCT #CancerTreatment #MultipleMyeloma #InductionTherapy #TandemTransplantation #ConsolidationTherapy #MaintenanceTherapy #ProteasomeInhibitors #Immunomodulators #CD38MonoclonalAntibodies #AlloHSCT #UHRMM #NovelTherapies
Expert Consensus on the Treatment of HRMM
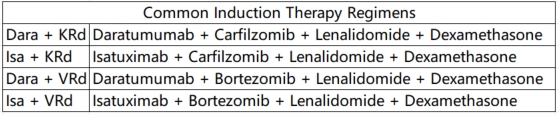
①Induction Therapy: For pre-transplantation induction therapy in HRMM, it is recommended to use a regimen based on CD38 monoclonal antibodies combined with proteasome inhibitors and immunomodulators. Recommended regimens include: Dara+KRd, Isa+KRd, Dara+VRd, and Isa+VRd. For patients who cannot tolerate a four-drug regimen, the KRd regimen is an alternative. For patients with significant extramedullary involvement (soft tissue or peripheral blood), additional cytotoxic drugs and, if necessary, radiotherapy can be added.
Therapy Regimens
②Auto-HSCT: Early auto-HSCT is the standard treatment for HRMM. For patients who receive auto-HSCT without significant adverse effects, tandem transplantation within six months post-transplant is recommended.
③Consolidation Therapy: For patients who do not undergo tandem transplantation, it is advised to continue consolidation therapy with the original induction regimen for 2–4 cycles.
④Maintenance Therapy: Maintenance therapy should involve a combination of proteasome inhibitors, immunomodulators, and CD38 monoclonal antibodies, either as dual or triple drug regimens. Therapy should continue until disease progression or intolerance.
⑤Clinical Research: Clinical studies targeting HRMM are encouraged, and it is recommended that HRMM patients prioritize enrollment in clinical trials.
**Treatment for Newly Diagnosed HRMM Not Suitable for Transplantation**
For MM patients not suitable for transplantation, an individualized treatment plan should be selected based on the patient’s fitness status score (IMWG GA score is recommended). For patients with good or moderate health, it is recommended to continue using the same regimen as for transplant-eligible patients. For frail patients, the VRd-lite (modified bortezomib + lenalidomide + dexamethasone) regimen and the DRd (daratumumab + lenalidomide + dexamethasone) regimen are currently the most commonly used first-line treatments.
**Treatment of Relapsed HRMM**
For patients with functional high risk and those defined as HRMM based on dynamic risk factors, re-induction regimens should include combinations of next-generation drugs or drugs with different mechanisms of action. Several clinical studies have shown that CAR-T cell (BCMA CAR-T) therapy can sustain efficacy in relapsed HRMM.
**Expert Consensus**
-
For relapsed HRMM patients, it is recommended to select combination regimens involving next-generation drugs or drugs with different mechanisms of action.
-
Patients with relapsed HRMM are encouraged to participate in clinical studies of CAR-T cell therapy or bispecific antibody immunotherapy.
**Summary**
For the treatment of HRMM, it is recommended to use multi-drug combination therapies with different mechanisms of action and bridge to auto-HSCT, aiming for deep and sustained MRD negativity and prolonging overall survival (OS) in patients. Currently, clinical trials of CAR-T cell therapy for newly diagnosed HRMM are being conducted, and combining auto-HSCT with CAR-T cell therapy can leverage the therapeutic benefits of both. Besides the BCMA target, CAR-T cell therapies targeting GPRC5D and FcRH5, as well as bispecific antibodies, have shown good efficacy in relapsed and refractory MM. New drugs and innovative diagnostic and therapeutic strategies, including immunotherapy, hold promise for overcoming the challenges in HRMM treatment.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HighRiskMultipleMyeloma #HRMM #MultipleMyeloma #CancerTreatment #Hematology #Oncology #MedicalResearch #CancerCare #CancerAwareness #ChinaMedicalAdvances #AutoHSCT #MedicalConsensus #UHRMM #BloodCancer
#CancerPrognosis #GeneticRiskFactors #CancerResearch #MMTreatment #MRD #Hematology #CancerSurvival #PrognosticFactors
#CancerCriteria #Hematology #HighRiskMyeloma #PlasmaCellLeukemia #CancerRelapse #CytogeneticAbnormalities
#InductionTherapy #TandemTransplantation #ConsolidationTherapy #MaintenanceTherapy #ProteasomeInhibitors #Immunomodulators #CD38MonoclonalAntibodies #AlloHSCT #NovelTherapies
