Tumor infiltrating lymphocyte (TIL) therapy is a type of immunotherapy that uses your body’s own cells to fight cancer. TIL (pronounced till) is a promising treatment for some types of solid tumors, especially melanoma.
What are tumor infiltrating lymphocytes (TILs)?
Lymphocytes (LIM-foh-sites) are a type of white blood cell. They’re an important part of your immune system. They help your body fight infections and diseases, including cancer.
Tumor infiltrating lymphocytes come from the tumor. Infiltrating (IN-fil-trey-ting) means to enter, and these lymphocytes are found inside the tumor.
The first step in TIL therapy is surgery to remove the tumor. Next, we separate the lymphocytes from the rest of the tumor cells. We send these lymphocytes to a laboratory where they’re isolated (separated from other types of cells) and grown in larger numbers.
How does TIL therapy for cancer work?
TIL therapy uses unaltered tumor cells to destroy the tumor itself. In TIL therapy, TILs are grown directly from the tissue removed during a surgical resection. They are then grown to very large numbers in a laboratory with interleukin-2 (IL-2), a protein that promotes rapid TIL growth, This process typically takes four to six weeks, which means that patients will qualify for TIL therapy only if their tumor is stable enough to withstand that waiting period.
Once successfully grown to billions in number, the TILs are infused back into the patient, where they actively attack cancer cells while leaving healthy cells alone. As with most other cellular immunotherapy approaches, a one-week course of chemotherapy is required before the TIL infusion to make space for the TIL to expand in the patient.
Unlike many other forms of cancer treatment that require ongoing care, TIL therapy is generally performed only one time. However, if a patient previously benefited from TIL therapy and he or she once again needs to undergo treatment, then TIL therapy may be performed a second time.

Approved TIL Therapy
Approved TIL Therapy therapies include:
| Approved drug | Approved drug indication |
|---|---|
| Amtagvi (lifileucel) |
|
Patient Stories
-
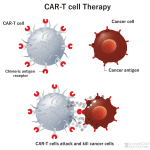
**Patient Story: CAR-T Therapy Brings New Hope for Lymphoma Patients – A Case from a Chinese Hospital** In the field of cancer treatment, China has achieved groundbreaking success with CAR-T cell therapy, especially in treating relapsed or refractory lymphoma. Today, we share the story of one patient whose life was transformed through this revolutionary treatment Read More
-
**Patient Story | Chinese CAR-T Therapy: An Inspiring Journey of Changing Fate for Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL)** #CAR-T #DLBCL #Lymphoma #CARTtherapy #RRDLBCL #Patientstory #CD19 In the field of cancer treatment, China has made remarkable strides in CAR-T cell therapy, especially in the fight against relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL). Read More