Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China Overcomes Solid Tumor Challenge: CAR-T Technology IM96 Leads Innovation in Colorectal Cancer Treatment
**China Overcomes Solid Tumor Challenge: CAR-T Technology IM96 Leads Innovation in Colorectal Cancer Treatment**

Colorectal Cancer
#IM96 #CART #ColorectalCancer #CARTtherapy #ESMO #2024ASCO #SolidTumor
China has made remarkable progress in the field of innovative cancer treatment. In October 2024, a Chinese biotechnology company announced that IM96 CAR-T cell injection, developed in collaboration with Peking University Cancer Hospital, received clinical trial approval from the National Medical Products Administration (NMPA). This marks a significant milestone for China in applying CAR-T technology to solid tumor treatment. IM96 is the first CAR-T cell drug approved in China for the treatment of metastatic colorectal cancer and has become the sixth CAR-T product approved for this company.
### Breakthroughs in IM96 Technology and Clinical Trial Highlights
IM96 utilizes ActSep-CD3/CD28 sorting and activation magnetic beads from China’s National Biopharmaceutical Technology Innovation Center. This is a cutting-edge technology, marking the first time China’s GMP-grade micron magnetic beads have been used in a CAR-T cell drug clinical trial, laying an important foundation for improved efficacy and safety.
The clinical trial of IM96 is led by Peking University Cancer Hospital, with Professor Lin Shen, a renowned oncology expert, as the principal investigator. Its preclinical study data has gained international recognition and was selected for the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, attracting significant attention.
### Exceptional Efficacy and Safety
IM96 has demonstrated outstanding efficacy in preclinical studies. Data shows a Disease Control Rate (DCR) of 73.7%, an Objective Response Rate (ORR) of 50%, and a median Progression-Free Survival (mPFS) exceeding 10 months. These results indicate a strong potential for IM96 in controlling tumor progression in metastatic colorectal cancer.
Additionally, IM96’s safety has been well-validated in preclinical studies, with only 5.0% of patients experiencing neurotoxicity or severe Cytokine Release Syndrome (CRS), underscoring its advantage in managing side effects.
### Meeting the Urgent Need for Metastatic Colorectal Cancer Treatment
Colorectal cancer is the third most prevalent cancer worldwide, with 1.93 million new cases and over 930,000 deaths each year. In China, the incidence and mortality rates of this cancer are high, particularly among advanced-stage metastatic patients who have limited treatment options. As an innovative solid tumor CAR-T drug developed independently in China, IM96 will address the gap in CAR-T therapies for colorectal cancer, offering new hope for patients.
### International Recognition and Academic Achievements
IM96 has been selected for consecutive presentations at the 2023 European Society for Medical Oncology (ESMO) and the 2024 ASCO Annual Meeting. Its excellent preclinical data has garnered global attention in the oncology community. The research findings have been accepted for publication in the prestigious *Journal for ImmunoTherapy of Cancer*. These achievements not only highlight China’s strength in CAR-T innovation but also contribute new perspectives and strategies to global cancer treatment.
The approval of IM96 marks a new phase in China’s development of CAR-T therapies, especially for solid tumors, and is expected to provide more effective and safer treatment options for colorectal cancer patients worldwide. This breakthrough will further enhance China’s international standing in cancer treatment and inject new momentum into global advancements in anti-cancer technologies.
🎉🎉To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #MedicalInnovation #ChinaBiotech #Oncology
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**2024 ASCO: National Institute’s New CAR-T Therapy CD7 CAR-T Cells, Multiple Blood Cancer Destroyers**
**2024 ASCO: National Institute’s New CAR-T Therapy CD7 CAR-T Cells, Multiple Blood Cancer Destroyers**

CAR-T Therapy
**2024 ASCO: Four Chinese-developed CAR-T therapies make a significant impact, targeting colorectal cancer, pancreatic cancer, and hematological tumors, with an overall response rate nearing 100.0%**
**CD7 CAR-T Cells: A Powerful Strike Against Hematological Tumors, Patients Achieve Complete Remission**
Patients with relapsed or refractory hematologic malignancies have limited treatment options and poor prognosis, with a 5-year overall survival rate of less than 20%. While allogeneic hematopoietic stem cell transplantation (HSCT) provides a critical strategy for treating aggressive hematologic cancers, HSCT treatment can also result in adverse reactions such as graft-versus-host disease (GVHD) and conditioning-related toxicities. Additionally, some patients with poor health cannot undergo this treatment. Therefore, new treatment methods are urgently needed, and the emergence of CAR-T therapy has brought new hope to patients with hematologic tumors.
The world-renowned journal, *The New England Journal of Medicine*, reported on a clinical study of “CD7 CAR-T cells for the treatment of relapsed or refractory CD7-positive hematologic tumors” (NCT04599556).
From November 2021 to September 2023, 10 patients with relapsed or refractory CD7-positive cancers were enrolled in the study, including 7 cases of acute myeloid leukemia (AML), 2 cases of T-cell acute lymphoblastic leukemia (ALL), and 1 case of T-cell lymphoblastic lymphoma (IVA stage). The median age was 56.5 years (range, 13.7–72.5 years). All patients had bone marrow involvement, with a median blast cell percentage of 36.0% (range, 2–87), and a median CD7 expression on blast cells of 93.0% (range, 80.7–97.7). All patients had received extensive prior treatments, with a median of 9.5 courses (range, 4–15 courses). After enrollment, patients first received an intensive lymphocyte-depleting regimen (cyclophosphamide, fludarabine, etoposide) and then CD7 CAR-T cell infusion therapy. After a median follow-up of 15.1 months, the results showed:
-
**Complete Remission (CR):** All patients (n=10) achieved complete remission (CR) after CAR-T cell therapy, though hematologic recovery was incomplete, with grade 4 pancytopenia. As of November 8, 2023 (data cutoff date), 6 patients had not received any further treatment and remained in minimal residual disease (MRD) negative complete remission.
-
**Overall Survival Rate:** The estimated 1-year overall survival rate was 68% [95% Confidence Interval (CI), 43–100].
-
**Disease-Free Survival Rate:** The estimated 1-year disease-free survival rate was 54% (95% CI, 29–100).
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#ASCO2024 #CARTherapy #BloodCancer #CD7CART #CancerResearch #Hematology #MedicalInnovation #CART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
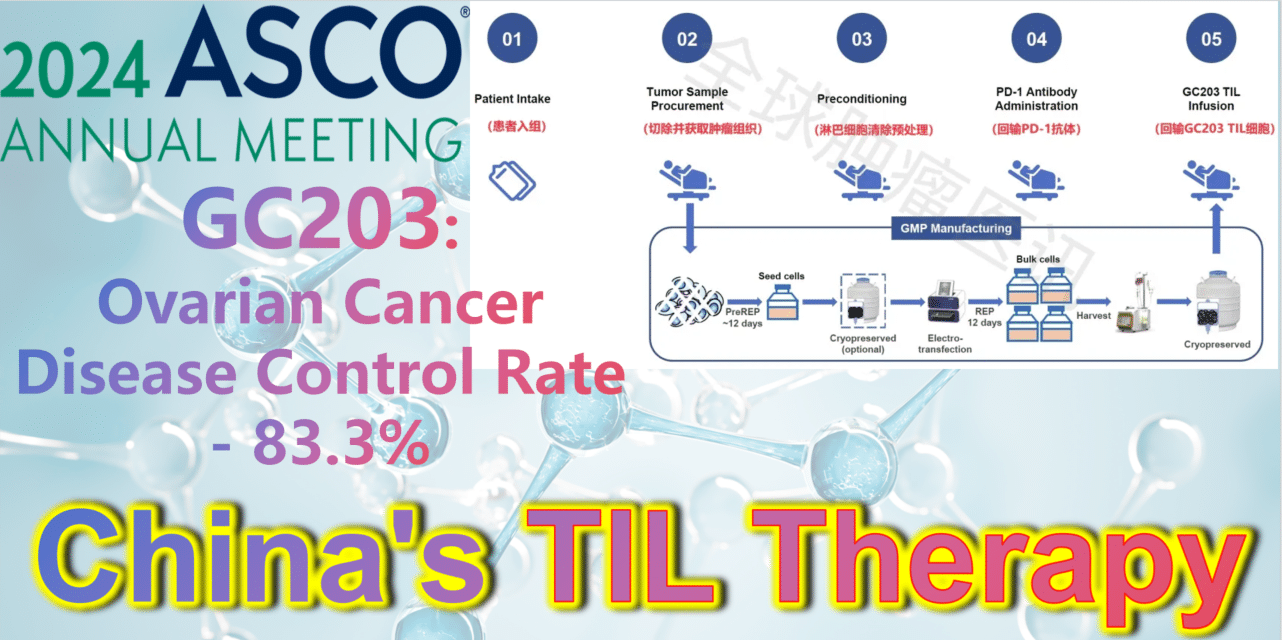
2024 ASCO China Voice: China’s TIL therapy – GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate
2024 ASCO China Voice: China’s TIL therapy makes a grand debut, targeting ovarian cancer.
**GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate**

TIL therapy
Ovarian cancer is a type of gynecologic tumor with a poor prognosis, with 70% of patients being diagnosed at a late stage. Unfortunately, effective treatment options for advanced ovarian cancer are quite limited, primarily relying on platinum-based chemotherapy. However, many ovarian cancer patients are not responsive to chemotherapy. Thus, there is an urgent need for new treatment options.
GC203 (mbIL-7-TIL) is a novel non-viral vector gene-modified TIL therapy utilizing membrane-bound IL-7. Developed by JunSai Biotech using the DeepTIL® cell expansion platform and NovaGMP® gene modification platform, it efficiently modifies T cells in a more economical way, enhancing the antitumor activity of TIL cells, activating internal immune cells, and avoiding systemic toxicity. It does not require lymphodepletion or combined IL-2 therapy post-infusion. A single patient is expected to save approximately 150,000 RMB in associated clinical costs, significantly improving the accessibility of TIL therapy and benefiting more cancer patients.
At the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, the latest clinical study results of GC203’s Phase 1 trial (NCT05468307) were announced. Between September 2021 and January 2024, 20 patients with recurrent ovarian cancer were enrolled, having undergone a median of 2.5 lines (range 1-9) of chemotherapy regimens (including PARP inhibitors, immune checkpoint inhibitors, etc.). After enrollment, patients first underwent tumor tissue resection, which was transported to GMP for a 22-26 day preparation period. The cryopreserved infusion products were then returned to the clinical center. Finally, patients received lymphocyte depletion pretreatment (including cyclophosphamide and hydroxychloroquine), a one-time PD-1 antibody infusion, and GC203 TIL cell reinfusion therapy.
After a median follow-up of 8.7 months (range, 2.9-18.8 months), results from 18 evaluable patients showed the following:
-
**Objective Response Rate (ORR):** The ORR in evaluable patients (n=18) was 33.3% (95% CI: 16.3%-56.3%). Among them, 22.2% (4 patients) achieved partial response (PR), and 11.1% (2 patients) achieved complete response (CR).
-
**Disease Control Rate (DCR):** The DCR in evaluable patients (n=18) was 83.3% (95% CI: 60.8%-94.2%).
-
**Median Progression-Free Survival (PFS):** The median PFS was 5.5 months (range, 1.0-14.1 months).
-
**Overall Survival (OS) Rate:** The 6-month OS rate was 75.6% (95% CI: 57.4%-99.6%); the 12-month OS rate was 68.8% (95% CI: 49.3%-95.9%).
-
**Adverse Reactions:** Most treatment-emergent adverse events (TEAEs) were grade 1 or 2, with common adverse reactions including elevated C-reactive protein levels (33%), fever (33%), and fatigue (11%), which could be alleviated or cured with symptomatic treatment. No other serious adverse reactions were observed.
In summary, for patients with recurrent or metastatic ovarian cancer with limited treatment options, GC203 TIL cell reinfusion therapy has shown good efficacy. Due to low-intensity pretreatment and no need for combined IL-2 therapy, its safety is significantly improved compared to traditional TIL therapy.
**How to Seek Help from TIL Therapy?**
The good news is that several TIL therapy clinical trials are currently recruiting in China, primarily targeting various solid tumors such as non-small cell lung cancer, melanoma, cholangiocarcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer, ovarian cancer, cervical cancer, endometrial cancer, fallopian tube cancer, urothelial cancer, and renal cancer.
Patients seeking help from TIL therapy can submit their complete treatment history, recent pathology reports, imaging examination reports, and discharge summaries to Advanced Medicine in China.
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#OvarianCancer #TILTherapy #CancerTreatment #ASCO2024 #GC203 #Immunotherapy #MedicalResearch #Biotech #Oncology #ClinicalTrials #CancerInnovation #JunSaiBiotech #TIL #CancerBreakthrough #PatientCare #MedicalAdvancements
