Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Real-World Performance of China’s Anti-Cancer Drug Zanubrutinib in CLL/SLL Treatment: Efficacy and Safety
**Real-World Performance of China’s Anti-Cancer Drug Zanubrutinib in CLL/SLL Treatment: Efficacy and Safety**

CLL
#Ibrutinib #Zanubrutinib #CLL #SLL #BTKinhibitor #Oncology #cancerdrug #BTK #ASCO
With the advent of BTK inhibitors, the treatment paradigm for chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) has undergone a complete transformation, moving away from traditional chemotherapy. Zanubrutinib, a powerful BTK inhibitor developed independently by BeiGene in China, is a next-generation Bruton’s tyrosine kinase inhibitor (BTKi) that has demonstrated remarkable efficacy in clinical trials. A study presented at the 2024 ASCO conference sheds light on the real-world treatment patterns and outcomes of zanubrutinib, providing valuable insights for patients and clinicians.
### **Study Background**
Zanubrutinib, a fully China-developed anti-cancer drug, launched in late 2019, has been shown to be more effective than the first-generation BTKi, ibrutinib, in CLL/SLL patients. This retrospective study analyzed patients treated with zanubrutinib between 2018 and 2023 at Kaiser Permanente Northern California, focusing on treatment patterns, adverse events (AEs), and survival outcomes in a real-world setting.
### **Study Findings**
The study analyzed 281 patients with key findings as follows:
– **Patient Profile**: The median age of patients was 71 years, with 64% being male. Most patients were white (75%), followed by African Americans (10%). Of the total, 190 patients switched from ibrutinib to zanubrutinib, while 91 patients were treated solely with zanubrutinib.
– **Adverse Events**: Whether patients switched from ibrutinib or started directly on zanubrutinib, the latter showed significantly lower rates of cardiac toxicity and treatment-limiting adverse events (TLAEs). The most common AEs for zanubrutinib included cytopenia and rashes/bruising, while ibrutinib was more often associated with atrial fibrillation and fatigue.
– **Dose Adjustments and Treatment Continuity**: Some patients adjusted their doses due to mild AEs, but the majority (79%) were still receiving zanubrutinib at the end of the study. While 13 patients died, there were no treatment-related deaths.
### **Study Conclusion**
This study demonstrates that regardless of prior ibrutinib use, China’s homegrown anti-cancer drug, zanubrutinib, exhibited excellent efficacy and safety. Compared to ibrutinib, zanubrutinib had a lower incidence of cardiac toxicity and maintained its effectiveness even in older patients with more comorbidities. These real-world data reinforce zanubrutinib’s pivotal role in the treatment of CLL/SLL, further validating its clinical application.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #RealWorldData #CancerResearch #BeiGene #ASCO2024
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Blood Cancer Solution Including leukemia, lymphoma, multiple myeloma, and others.
 Blood Cancer Solution
Blood Cancer Solution
 Including leukemia, lymphoma, multiple myeloma, and others.
Including leukemia, lymphoma, multiple myeloma, and others.

Blood Cancer
#leukemia #lymphoma #multiplemyeloma
#Hematologic malignancies are a group of malignant diseases originating from hematopoietic cells, often affecting the bone marrow, blood, and various organs and tissues throughout the body. Common types of hematologic malignancies include leukemia, myelodysplastic syndromes, lymphomas, multiple myeloma, and myeloproliferative neoplasms.
The causes of these diseases are complex, involving genetic mutations, immune abnormalities, radiation exposure, contact with harmful chemicals, infections, and hereditary factors. Additionally, poor lifestyle habits, high levels of stress, and environmental factors can also increase the risk of developing these conditions.
With an aging population and advancements in medical technology, the incidence of hematologic malignancies has been rising globally. In China, the incidence and mortality rates of leukemia and lymphoma are now among the top ranks of all malignancies.
However, hematologic malignancies are not incurable. In recent years, the treatment methods for these diseases have seen significant progress. From traditional combination chemotherapy and radiotherapy to hematopoietic stem cell transplantation, monoclonal antibody therapy, antibody-drug conjugates, small molecule targeted therapies, and the latest immunotherapies, treatment options have become increasingly diverse and precise.
Combination chemotherapy remains a primary treatment for many hematologic malignancies, despite its significant side effects. The efficacy of these treatments cannot be ignored. Modern chemotherapy regimens are continually being refined, including the incorporation of new cytotoxic drugs and targeted therapies, as well as the use of monoclonal antibodies. Additionally, the appropriate use of antiemetics, hematopoietic growth factors, and anti-infective agents helps to mitigate adverse effects.
Hematopoietic stem cell transplantation continues to be one of the most effective treatments for certain hematologic malignancies. The development of this treatment in China has been rapid, with 170 registered transplant centers by 2020.
Monoclonal antibodies, often referred to as “biological missiles,” have a high degree of specificity and single biological activity. They have revolutionized the treatment of hematologic malignancies. Antibody-drug conjugates (ADCs) utilize monoclonal antibodies to accurately identify tumor cell markers, guiding the delivery of chemotherapy drugs for targeted treatment.
Small molecule targeted therapies work by interfering with specific genes or proteins to inhibit tumor cell growth and proliferation. Gleevec, the first small molecule targeted therapy, increased the five-year survival rate for chronic myeloid leukemia (CML) patients from 30% to 89%, marking a breakthrough in cancer treatment. Today, there are numerous small molecule targeted drugs available for the treatment of hematologic malignancies, including BCR-ABL inhibitors, BTK inhibitors, BCL-2 inhibitors, PI3K inhibitors, and XPO1 inhibitors, with many more drugs currently in clinical trials expected to become available soon.
Immunotherapy includes immune checkpoint inhibitors (such as PD-1/L1), cancer vaccines, cellular immunotherapies (such as #CART), and nonspecific immunomodulatory treatments. #CARTtherapy, in particular, has gained widespread attention as an emerging curative treatment. This approach involves extracting a patient’s T cells, modifying them outside the body to specifically recognize and attack tumor cells, and then reinfusing the modified T cells into the patient. This therapy has been successfully applied to various hematologic malignancies, including acute lymphoblastic leukemia, lymphomas, and multiple myeloma. The first patient treated with CAR-T therapy has been disease-free for 11 years.
In recent years, China has made significant advances in the treatment of hematologic malignancies. The establishment of the “Chinese Expert Consensus on the Diagnosis and Treatment of High-Risk Multiple Myeloma” and the presentation by Professor Huang He at the 2024 #EHA conference on targeting CD7 universal CAR-T therapy for T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) have shown remarkable efficacy and safety. Additionally, exciting new data from the 2024 American Society of Clinical Oncology (#ASCO) annual meeting highlighted the efficacy of Relma-cel in treating relapsed/refractory large B-cell lymphoma (R/R LBCL), with a four-year overall survival rate (#OS) of 66.7%. Particularly noteworthy is the research on multiple myeloma, where the BCMA-targeted CAR-T therapy has demonstrated deep and lasting responses, with a complete response (#CR) rate of 82.4% and a 12-month progression-free survival (#PFS) rate of 85.5%.
With the continuous development of new treatments and the emergence of new drugs, hematologic malignancies in China are no longer considered incurable diseases. Through standardized, individualized, and precise treatments, many patients with hematologic malignancies can achieve long-term disease-free survival, and even a cure, returning to normal work and life. As medicine continues to advance, every life will continue to shine brightly!

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#HematologicMalignancies #LeukemiaAwareness #LymphomaResearch #MultipleMyeloma #BloodCancer #CancerResearch #CAR_Therapy #StemCellTransplant #Immunotherapy #TargetedTherapy #MonoclonalAntibodies #CancerTreatment #MedicalAdvancements #CancerSurvivor #HealthcareInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
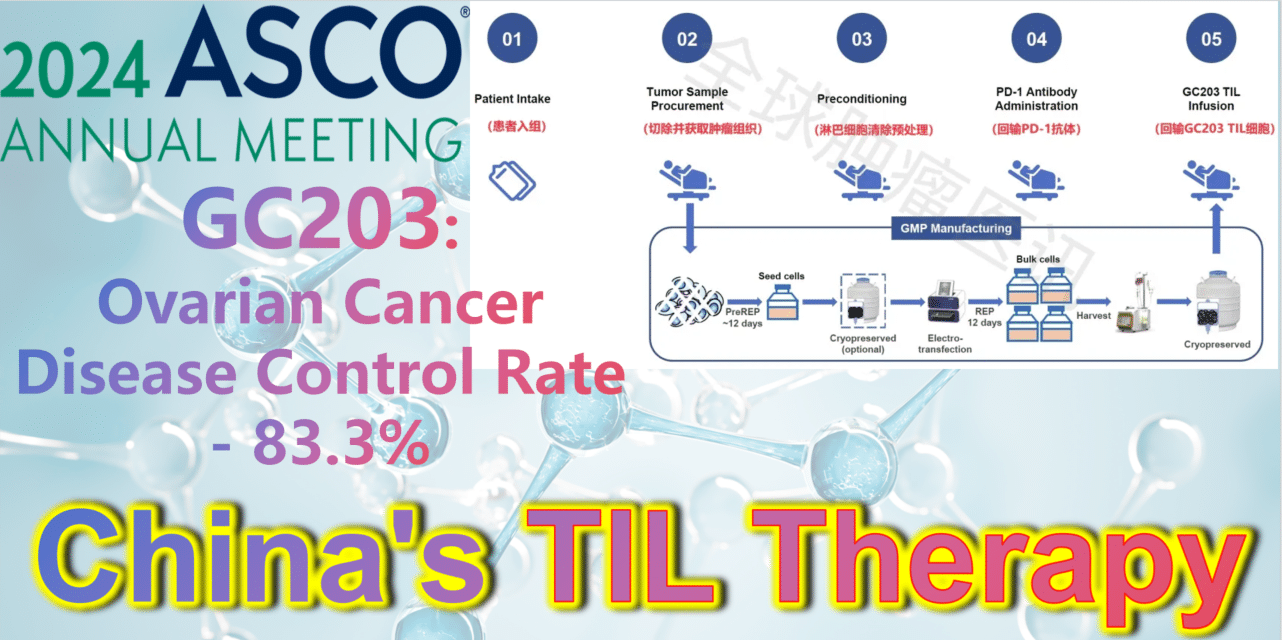
2024 ASCO China Voice: China’s TIL therapy – GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate
2024 ASCO China Voice: China’s TIL therapy makes a grand debut, targeting ovarian cancer.
**GC203: A Powerful Strike Against Ovarian Cancer with an 83.3% Disease Control Rate**

TIL therapy
Ovarian cancer is a type of gynecologic tumor with a poor prognosis, with 70% of patients being diagnosed at a late stage. Unfortunately, effective treatment options for advanced ovarian cancer are quite limited, primarily relying on platinum-based chemotherapy. However, many ovarian cancer patients are not responsive to chemotherapy. Thus, there is an urgent need for new treatment options.
GC203 (mbIL-7-TIL) is a novel non-viral vector gene-modified TIL therapy utilizing membrane-bound IL-7. Developed by JunSai Biotech using the DeepTIL® cell expansion platform and NovaGMP® gene modification platform, it efficiently modifies T cells in a more economical way, enhancing the antitumor activity of TIL cells, activating internal immune cells, and avoiding systemic toxicity. It does not require lymphodepletion or combined IL-2 therapy post-infusion. A single patient is expected to save approximately 150,000 RMB in associated clinical costs, significantly improving the accessibility of TIL therapy and benefiting more cancer patients.
At the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, the latest clinical study results of GC203’s Phase 1 trial (NCT05468307) were announced. Between September 2021 and January 2024, 20 patients with recurrent ovarian cancer were enrolled, having undergone a median of 2.5 lines (range 1-9) of chemotherapy regimens (including PARP inhibitors, immune checkpoint inhibitors, etc.). After enrollment, patients first underwent tumor tissue resection, which was transported to GMP for a 22-26 day preparation period. The cryopreserved infusion products were then returned to the clinical center. Finally, patients received lymphocyte depletion pretreatment (including cyclophosphamide and hydroxychloroquine), a one-time PD-1 antibody infusion, and GC203 TIL cell reinfusion therapy.
After a median follow-up of 8.7 months (range, 2.9-18.8 months), results from 18 evaluable patients showed the following:
-
**Objective Response Rate (ORR):** The ORR in evaluable patients (n=18) was 33.3% (95% CI: 16.3%-56.3%). Among them, 22.2% (4 patients) achieved partial response (PR), and 11.1% (2 patients) achieved complete response (CR).
-
**Disease Control Rate (DCR):** The DCR in evaluable patients (n=18) was 83.3% (95% CI: 60.8%-94.2%).
-
**Median Progression-Free Survival (PFS):** The median PFS was 5.5 months (range, 1.0-14.1 months).
-
**Overall Survival (OS) Rate:** The 6-month OS rate was 75.6% (95% CI: 57.4%-99.6%); the 12-month OS rate was 68.8% (95% CI: 49.3%-95.9%).
-
**Adverse Reactions:** Most treatment-emergent adverse events (TEAEs) were grade 1 or 2, with common adverse reactions including elevated C-reactive protein levels (33%), fever (33%), and fatigue (11%), which could be alleviated or cured with symptomatic treatment. No other serious adverse reactions were observed.
In summary, for patients with recurrent or metastatic ovarian cancer with limited treatment options, GC203 TIL cell reinfusion therapy has shown good efficacy. Due to low-intensity pretreatment and no need for combined IL-2 therapy, its safety is significantly improved compared to traditional TIL therapy.
**How to Seek Help from TIL Therapy?**
The good news is that several TIL therapy clinical trials are currently recruiting in China, primarily targeting various solid tumors such as non-small cell lung cancer, melanoma, cholangiocarcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer, ovarian cancer, cervical cancer, endometrial cancer, fallopian tube cancer, urothelial cancer, and renal cancer.
Patients seeking help from TIL therapy can submit their complete treatment history, recent pathology reports, imaging examination reports, and discharge summaries to Advanced Medicine in China.
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#OvarianCancer #TILTherapy #CancerTreatment #ASCO2024 #GC203 #Immunotherapy #MedicalResearch #Biotech #Oncology #ClinicalTrials #CancerInnovation #JunSaiBiotech #TIL #CancerBreakthrough #PatientCare #MedicalAdvancements
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
The Hidden “Anti-Cancer Weapon” in Tumor Tissue – TIL Cells
### The Hidden “Anti-Cancer Weapon” in Tumor Tissue – TIL Cells

TIL Therapy
