Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China`s Breakthrough TIL Therapy GT201 Shows Remarkable Results in Advanced Solid Tumor Treatment: 100% Disease Control Rate, 69% Tumor Reduction
**China`s Breakthrough TIL Therapy GT201 Shows Remarkable Results in Advanced Solid Tumor Treatment: 100% Disease Control Rate, 69% Tumor Reduction**

TIL Therapy
#TILTherapy #GT201 #SolidTumor #TIL #ASCO2024
Recent research results indicate that the next-generation gene-edited TIL (Tumor-Infiltrating Lymphocyte) therapy, GT201, independently developed by Chinese biotech company GRIT Biotechnology, has shown significant potential in combating various advanced solid tumors. Through a unique membrane-bound cytokine design and the advanced retroviral system StaViral, GT201 has successfully overcome the traditional TIL therapy`s excessive reliance on IL-2, significantly improving its efficacy and durability in cancer treatment.
At the 2024 ASCO conference, the research team presented the results of the Phase I clinical trial for GT201. A total of 7 patients with advanced solid tumors, including non-small cell lung cancer, malignant melanoma, cervical cancer, and ovarian cancer, were enrolled in the trial. These patients had previously undergone at least three different treatments and showed notable efficacy after receiving GT201.
The data revealed that, among the 7 evaluable patients, 3 (42.9%) achieved confirmed partial responses (PR), with one patient’s tumor shrinking by an impressive 69%. Additionally, 2 patients (28.6%) achieved stable disease (SD). Notably, all 3 patients in the non-small cell lung cancer subgroup achieved disease control (SD ≥ 24 weeks or PR), resulting in a 100% disease control rate. These findings not only highlight the efficacy of GT201 but also bring new hope to patients with advanced non-small cell lung cancer.
Another significant advantage of GT201 is its safety profile. Throughout the trial, the therapy demonstrated good tolerability with manageable side effects. This result suggests that GT201 has the potential to become a powerful and effective treatment option for patients with advanced solid tumors, especially in the field of non-small cell lung cancer, where its objective response rate and disease control durability are highly encouraging.
As the clinical trials of GT201 continue to progress, it is poised to lead the future of solid tumor treatment and further advance TIL therapies. We look forward to more patients benefiting from this groundbreaking treatment.
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#SolidTumorTreatment #NonSmallCellLungCancer #CancerImmunotherapy #GRITBiotechnology #CancerResearch #GeneEditing #CancerTreatmentInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

2024 ASCO | Relma-cel Achieves a 4-Year OS Rate of 66.7% in Treating R/R LBCL, Making Lymphoma Cure Possible
2024 ASCO | Relma-cel Achieves a 4-Year OS Rate of 66.7% in Treating R/R LBCL, Making Lymphoma Cure Possible

Lymphoma
Over the past few decades, there have been significant advancements in the treatment of hematologic malignancies, particularly with CAR-T cell therapy. Recently, Relma-cel (relmacabtagene autoleucel) showcased promising new data at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, highlighting its remarkable efficacy in treating relapsed/refractory large B-cell lymphoma (R/R LBCL).
### Breakthrough in CAR-T Therapy
Relma-cel is a CD19-targeted CAR-T cell therapy that reprograms a patient’s own T cells to recognize and attack cancer cells. Studies have shown that Relma-cel achieves a 4-year overall survival (OS) rate of 66.7% in patients with R/R LBCL, with the rate soaring to 82.5% for those who achieve complete remission (CR). These results indicate that Relma-cel not only significantly extends survival but also increases the cure rate for these patients.
### The Power of Long-Term Follow-Up Data
The RELIANCE study, one of the largest CAR-T cell therapy studies in China, aims to evaluate the efficacy and safety of Relma-cel in R/R LBCL patients. The latest 4-year follow-up data reveals that 25 patients remain alive, and the median overall survival (OS) has not yet been reached. This milestone offers immense hope for patients and physicians, underscoring the long-term survival benefits of this therapy.
### Balancing Safety and Efficacy
Relma-cel also excels in safety. The incidence rates of ≥3 grade cytokine release syndrome (CRS) and neurotoxicity (NT) are 5.1% and 3.4%, respectively. Compared to other CD19 CAR-T cell therapies, Relma-cel effectively reduces side effects while significantly enhancing efficacy. This success is largely attributed to its unique CAR structure design, which includes a 4-1BB costimulatory domain, a CD28 transmembrane domain, and an optimized hinge region. These features enable Relma-cel to survive longer in the body and enhance its tumor-killing power.
### Future Prospects
As Relma-cel continues to expand its reach, more R/R LBCL patients are expected to benefit from this advanced treatment. Currently, Relma-cel is exploring its potential use in second-line treatment for LBCL and frontline therapy for high-risk LBCL patients. Additionally, it has shown excellent clinical value in treating relapsed/refractory follicular lymphoma (R/R FL) and mantle cell lymphoma (R/R MCL).
Relma-cel’s success not only brings new hope to lymphoma patients but also signifies a new era in the treatment of hematologic malignancies. We look forward to more research findings on Relma-cel, which will bring hope and possibilities for curing lymphoma to patients worldwide.
With Relma-cel, curing lymphoma is no longer an unattainable dream. Let’s anticipate more breakthroughs in this field, bringing new light to the lives of every patient.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#LymphomaTreatment #CAR_TTherapy #CancerResearch #RelmaCel #HopeForPatients #MedicalAdvancements #ASCO2024 #InnovativeTherapies #Survivorship #CureLymphoma
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
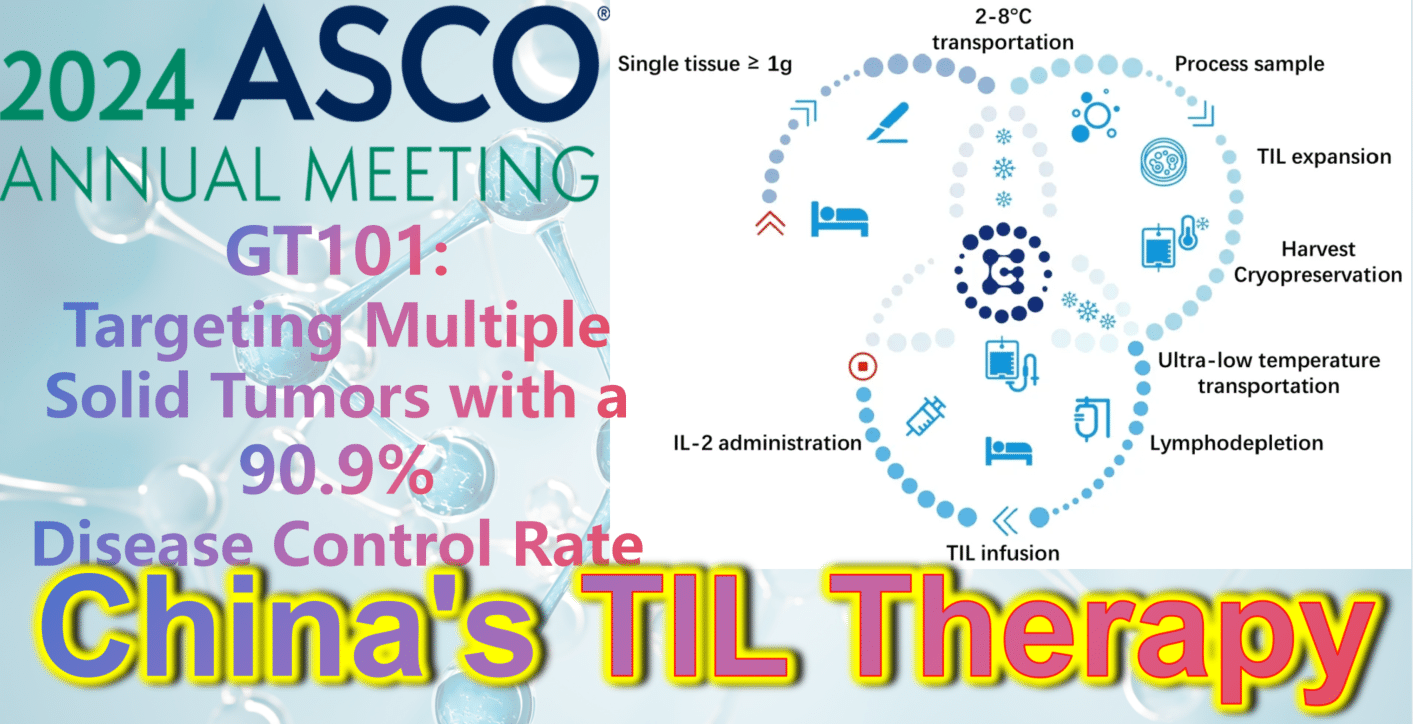
2024 ASCO China Highlights: China’s Indigenous TIL Therapy – GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate
**2024 ASCO China Highlights: China’s Indigenous TIL Therapy Makes a Strong Debut, Targeting Small Cell Lung Cancer, Melanoma, Cervical Cancer, with a DCR Over 90%**
**GT101: Targeting Multiple Solid Tumors with a 90.9% Disease Control Rate**

TIL Therapy
