Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Breakthrough Advances in CAR-T Cell Therapy Combined with TKI for Malignant Hematologic System Tumors
Breakthrough Advances in CAR-T Cell Therapy Combined with TKI for Malignant Hematologic System Tumors

At this year’s ASH conference, Professor Huang He, the director of the First Affiliated Hospital of Zhejiang University School of Medicine, presented a significant study on CAR-T cell therapy for B-ALL, marking a groundbreaking stride in the treatment of malignant hematologic system tumors.
This study, targeting newly diagnosed adult Ph-positive acute lymphoblastic leukemia, investigated the combined treatment of dasatinib and CAR-T cell therapy as a frontline therapy for initial patients. The integration of CAR-T cell therapy with tyrosine kinase inhibitors (TKI) aimed to enhance treatment effectiveness.
The study revealed promising outcomes: among 18 patients, the complete molecular remission rate after CD19 CAR-T cell therapy reached 72.2%. Among these patients, subsequent treatment with CD22 CAR-T cells achieved a complete molecular remission rate of 76.9%. At a median follow-up of 13.5 months post CAR-T cell therapy, 16 patients maintained complete hematological remission, while 14 patients sustained complete molecular remission without undergoing allogeneic transplantation. Notably, no CRS (cytokine release syndrome) ≥ Grade 3 or ICANS (immune effector cell-associated neurotoxicity syndrome) occurred during CAR-T cell therapy.

Professor Huang He’s team’s groundbreaking research presented at the ASH conference signifies a leap forward in the treatment of blood-related cancers, signaling a more efficient and accessible era. These findings instill hope for refining treatment strategies and potentially making a significant impact in the field of hematologic oncology.

Beyond the groundbreaking progress in CAR-T cell therapy combined with TKI, the team showcased seven oral presentations and 26 poster exhibitions. These studies encompassed fundamental research on blood disorders, leukemia pathogenesis, acute lymphoblastic leukemia, acute myeloid leukemia, lymphoma, myeloma, hematopoietic stem cell transplantation, and cellular immunotherapy, all crucial for enhancing clinical practices.
We eagerly anticipate further groundbreaking strides by Chinese research teams in cancer treatment studies, anticipating the transformative impact of these research findings in the future.
#ASHAnnualMeeting #HematologyResearch #CARTCellTherapy #StemCellTransplantation #BloodCancer #ClinicalInnovation #OncologyAdvancements #Cancertherapy #MedicalResearch #ASH #CART #CARTTherapy #CARTCell #TKI #tyrosinekinaseinhibitors
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Research Team New Treatment Shows Promise: CD7 CAR-T Cell Therapy for Relapsed/Refractory AML
Chinese Research Team New Treatment Shows Promise: CD7 CAR-T Cell Therapy for Relapsed/Refractory AML

A recent clinical trial has unveiled a new prospect for treating relapsed/refractory acute myeloid leukemia (AML). The study delved into the safety and efficacy of a natural selection CD7 CAR-T cell (NS7 CAR-T cell) in treating CD7+ relapsed/refractory AML patients.
The research involved 10 cases of CD7+ relapsed/refractory AML, where 30% of AML patients expressed CD7. Following treatment with NS7 CAR-T cell infusion, the results showed that within 4 weeks post-infusion, 70% of patients achieved complete bone marrow remission (CR), with 6 patients achieving a deep remission at the level of minimal residual disease (MRD). Notably, the loss of CD7 was identified as a primary reason for non-response in some patients.
The study also indicated that the majority of patients (80%) experienced mild cytokine release syndrome (CRS) post-infusion, with no observed neurotoxicity.
Furthermore, for select patients, continuing with a second allogeneic hematopoietic stem cell transplantation post NS7 CAR-T cell treatment resulted in promising long-term outcomes. However, further exploration is required for patients who did not undergo consolidative transplants to enhance treatment efficacy.
Professor Lu Peihua(Lu Daopei Hospital) remarked that this study highlights the potential of CD7 CAR-T cells as a bridging therapy before transplantation, demonstrating preliminary therapeutic efficacy for relapsed/refractory AML patients. Nevertheless, a comprehensive evaluation of its efficacy requires a larger sample size and longer-term follow-up.

This preliminary study represents a significant advancement in the field of AML treatment, but collaborative efforts and more research are still necessary. Looking ahead, we anticipate further studies and treatment outcomes to bring more beneficial therapeutic options for the long-term survival of patients, supported by a broader patient cohort and collaborative efforts from the medical community.
The outlook for this CD7 CAR-T cell therapy is promising, especially for AML patients who have undergone various treatments and allogeneic hematopoietic stem cell transplants. We look forward to more in-depth research and treatment outcomes in the future, offering more beneficial treatment choices for long-term survival among patients.
(source: ClinicalTrials.gov registration number NCT04938115
Preliminary results published by Professor Lu Peihua and their team in a top-tier journal)

#AMLResearch #CancerTreatment #CARTTherapy #Leukemia #MedicalBreakthrough #Cancer #AML #Immunotherapy #ClinicalTrials #BloodCancer #PatientCare #HealthcareAdvances #PrecisionMedicine #ScienceAndHealth #MedicalScience #CancerSurvivor #AMLWarriors #ResearchProgress #CancerFree #HopeInTreatment #acutemyeloidleukemia
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
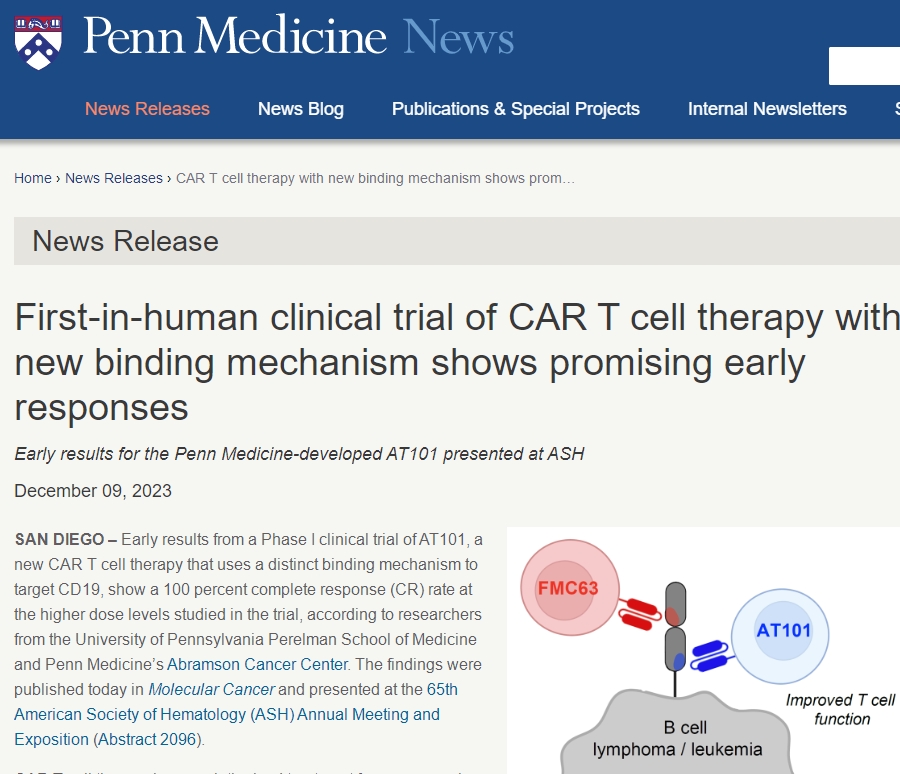
New Generation CAR-T Cell Therapy AT101: Phase I Clinical Trial Shows 100% Complete Remission Rate!
New Generation CAR-T Cell Therapy AT101: Phase I Clinical Trial Shows 100% Complete Remission Rate!

Researchers recently released exciting news: the Phase I clinical trial of their newly developed CAR-T cell therapy, AT101, demonstrated a 100% complete remission rate among patients receiving high-dose treatment. This groundbreaking achievement was published in the latest “Molecular Cancer” journal and gained significant attention at the 65th American Society of Hematology (ASH) Annual Meeting.
Despite recent FDA investigations into the safety of CAR-T cell therapy, it remains the most promising choice for blood cancer patients who have tried other treatment methods unsuccessfully. CAR-T cell therapy has fundamentally changed the treatment landscape for many blood cancer patients. While some patients show long-term responses to it, others do not.
The new CAR-T cell therapy, AT101, developed by researchers from the Perelman School of Medicine and the Abramson Cancer Center at the University of Pennsylvania, exhibited highly encouraging results due to its design targeting a new epitope of CD19 through a unique binding mechanism. Most currently approved CD19 CAR-T cell therapies target the same epitope, yet many patients eventually relapse. AT101, by targeting a different CD19 epitope, shows faster action rates and aims to reduce the failure rate of CAR-T cell therapy while improving clinical efficacy.
In this Phase I clinical trial, 14 patients with relapsed or refractory B-cell non-Hodgkin lymphoma (NHL) received treatment. Nine out of twelve patients achieved a complete remission status, indicating a total remission rate of 91.7%, with eight patients achieving complete remission. These patients did not experience cancer relapse, and the drug showed promising safety results.
While further research and larger-scale clinical trials are necessary, these early results instill considerable confidence. The success of AT101 holds promise for bringing new hope to blood cancer patients, especially those who previously did not receive effective CAR-T cell therapy. This study also aims to expand to a broader patient population, offering more treatment options and possibilities.
#CARTTherapy #BloodCancerTreatment #AT101Research #CancerRemission #MedicalBreakthrough #ASHAnnualMeeting #ClinicalTrialSuccess #UniversityResearch #CancerResearchUpdate #HopeForPatients #AT101 #BloodCancer #CancerTreatment
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Chinese Research: Hopes of CAR-T in Second-Line Treatment of LBCL(Lymphoma)
Diffuse Large B-cell Lymphoma (LBCL)is a heterogeneous malignant tumor of the blood system, with approximately 40% of LBCL patients experiencing relapse or developing resistance after first-line treatment, progressing to relapsed/refractory large B-cell lymphoma (R/R LBCL). In recent years, chimeric antigen receptor T-cell therapy targeting CD19 (CAR-T) has made significant breakthroughs in treating relapsed or refractory B-cell non-Hodgkin lymphoma.

At the 65th American Society of Hematology (ASH) Annual Meeting held from December 9th to 12th this year in the Eastern United States, important real-world evidence of CAR-T cell therapy as a second-line standard treatment for R/R LBCL was presented in the form of a poster (ASH Abstract #4876), providing crucial evidence-based medicine for the clinical application of CAR-T.
CAR-T Cell Therapy as Second-Line Treatment for R/R LBCL: Real-World
Evidence Among 110 patients receiving CAR-T infusion, the overall response rate (ORR) was 82.7%, and the complete response (CR) rate was 61.8%. Median progression-free survival (PFS) and overall survival (OS) were not reached. Estimated 6-month PFS and OS rates were 64.1% (95% CI, 52.8%-73.4%) and 84.4% (95% CI, 73.8%-90.9%), respectively.

The advent of CAR-T cell therapy has brought hope of cure for R/R LBCL patients. Based on its critical research and outstanding real-world application data, CAR-T cell therapy has gained recommendations from authoritative guidelines at home and abroad, and has become a crucial choice for second-line treatment in R/R LBCL patients.
#CARTCellTherapy #CancerTreatment #ScienceInnovation #GeneticMedicine #TumorTreatment #HealthcareTech #MedicalScience #CancerAwareness #PatientCare #FutureOfMedicine #CART #LBCL #Lymphoma #bloodcancer
