Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
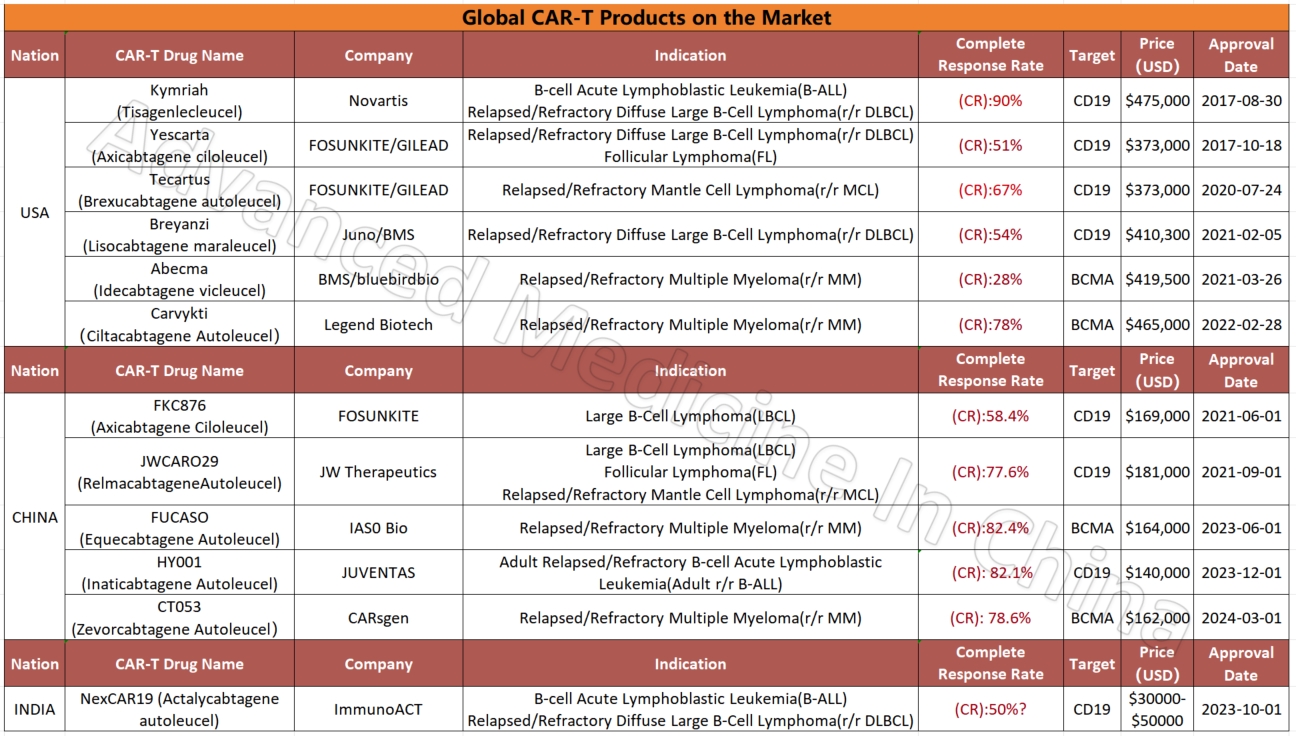
Global CAR-T Products on the Market and BCMA-Targeted CAR-T Products
Global CAR-T Products on the Market

CAR-T
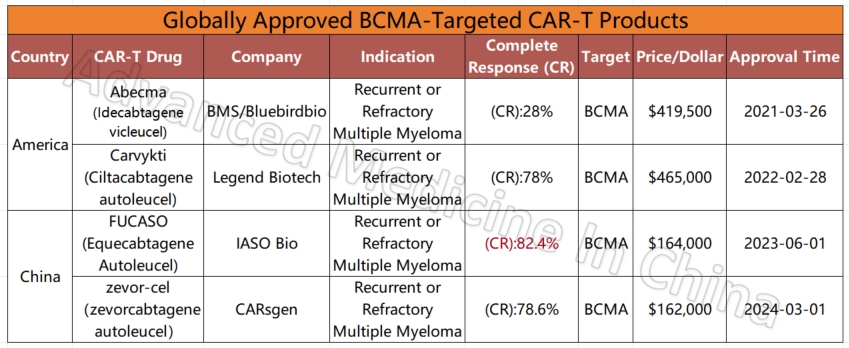
Globally Approved BCMA-Targeted CAR-T Products
– Multiple Myeloma
Multiple Myeloma

 To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of Advanced Medicine in China for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**
**Chinese Medical Team Releases Dual-Target CAR-T Data: 100% Complete Remission Rate in High-Risk MM Patients**

High-Risk MM
#CARTTherapy #MultipleMyeloma #BCMA #CD19 #JAMAOncology #MM #HRMM #DualTarget
Once again, a Chinese medical team is at the forefront of innovation, making significant strides in the field of CAR-T cell therapy. A recent study published in *JAMA Oncology* (B-Cell Maturation Antigen/CD19 Dual-Targeting Immunotherapy in Newly Diagnosed Multiple Myeloma) revealed remarkable results for the dual-target BCMA/CD19 CAR-T therapy in high-risk newly diagnosed multiple myeloma (NDMM) patients. In this study, all 19 participants achieved stringent complete remission (sCR) and were found to be minimal residual disease (MRD) negative, resulting in an astounding 100% remission rate.
**CAR-T Therapy: A Breakthrough in Hematologic Cancer Treatment**
Multiple myeloma (MM) is a challenging hematologic cancer, particularly for patients with high-risk features, where traditional treatments often show limited efficacy. CAR-T cell therapy is an innovative immunotherapy that reprograms a patient’s own T cells to recognize and attack cancer cells. In recent years, this therapy has shown significant success in treating leukemia, lymphoma, and relapsed/refractory MM (RRMM). The latest study further demonstrates that dual-target CAR-T therapy, focusing on BCMA and CD19, can significantly improve the prognosis for high-risk MM patients.
**The Impressive Results of China’s Dual-Target CAR-T Therapy**
The CAR-T therapy used in this study, GC012F, targets both B-cell maturation antigen (BCMA) and CD19 surface antigens, showing powerful anti-tumor effects. Every patient in the study not only achieved complete remission after treatment but also maintained a long-term MRD-negative status, meaning that traces of cancer cells were nearly undetectable post-therapy.
More importantly, the study highlighted the swift effectiveness of the Chinese GC012F therapy—patients reached their first complete remission in a median time of just 84 days, with MRD-negative status achieved in as little as 28 days. This rapid anti-tumor response provides a crucial treatment window for patients, significantly improving their prognosis.
**Safety and Future Prospects**
In addition to its impressive efficacy, GC012F demonstrated favorable safety. Only 27% of patients experienced mild to moderate cytokine release syndrome (CRS), and no cases of immune effector cell-associated neurotoxicity syndrome (ICANS) were observed. This is in stark contrast to previous studies in relapsed/refractory patients, where CRS rates were higher, highlighting the safety advantage of this therapy in newly diagnosed patients.
Though the study sample size was small, these findings bring new hope for high-risk multiple myeloma patients. As larger clinical trials are conducted and combination therapies are explored, this therapy has the potential to offer better survival outcomes for more patients.
**Conclusion**
China’s CAR-T cell therapy research continues to lead the way in hematologic cancer treatment. The success of the BCMA/CD19 dual-target CAR-T therapy not only demonstrates its strong efficacy in high-risk multiple myeloma but also provides a new direction for treating hematologic cancers globally. As research deepens, we anticipate that this groundbreaking therapy will bring new life-saving opportunities to more patients.
This breakthrough in Chinese CAR-T therapy represents not only scientific progress but also underscores China’s pivotal role in the global fight against cancer.
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
WhatsApp: Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #MedicalInnovation #CancerResearch #ChinaHealthcare #HematologicCancer #CancerBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The significance of the 11 CD19 and BCMA CAR-T therapies currently approvedin China and the U.S. forpatients with hematologic malignancies.
The significance of the 11 CD19 and BCMA CAR-T therapies currently approvedin China and the U.S. forpatients with hematologic malignancies.
目前中美上市的11款CD19和BCMA CAR-T对血液瘤患者的意义
Expert:
**Jing Pan**
**Associate Chief Physician**
**Department of Pediatric Hematology, Beijing Gaobo Hospital**
**Professional Memberships:**
– Member, Pediatric Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Hematologic Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Clinical Application Professional Committee of the Chinese Medical Biotechnology Association
**Expertise:**
Dr. Pan currently manages an 80-bed pediatric hematology unit and has extensive experience in pediatric hematology, particularly in CAR-T cell immunotherapy, with nearly 10 years of experience in the field. She is dedicated to stratified CAR-T treatment, optimizing the management of complications during CAR-T therapy, and establishing an efficacy monitoring system post-treatment. Dr. Pan and her team have accumulated one of the largest single-center case collections globally, particularly in the areas of sequential CAR-T therapy for improving long-term outcomes in B-ALL and in exploring autologous and allogeneic CD5, CD7 CAR-T therapy for T-ALL/LBL.
Her related clinical research on CD7 CAR-T, CD19 CAR-T, CD22 CAR-T, and CD19-22 sequential CAR-T has been published in leading international journals such as *Lancet Oncology*, *JCO*, *Blood*, *JHO*, and *Leukemia*. Additionally, she has frequently presented the latest advancements in her team’s immunotherapy research at both domestic and international conferences, including ASCO, ASH, EHA, and JSH.
潘 静
副主任医师
北京高博医院 小儿血液科
中国抗癌协会小儿肿瘤专业委员会专业委员
中国抗癌协会血液肿瘤专业委员会青年委员
中国医药生物技术协会医药生物技术临床应用专业委员会青年委员
擅长:
目前独立管理床位数80张的儿童血液病区。从事儿童血液科临床工作多年,特别是在儿童CAR-T细胞免疫治疗方面积累了近10年的经验。致力于CAR-T的分层治疗,优化CAR-T治疗过程中的并发症处理,建立CAR-T治疗后的疗效监控体系。尤其是其带领团队在序贯CAR-T提高B-ALL远期预后,T-ALL/LBL的自体、异体CD5、CD7CAR-T治疗探索方面目前积累了全球较大的单中心病例数。相关的CD7CAR-T临床研究、CD19CAR-T临床研究、CD22CAR-T临床研究、CD19-22序贯CAR-T研究等,发表在国际血液病权威杂志期刊Lancet on-cology、JC0、Blood、JHO和Leukemia。并多次在国内外学术会议(美国临床肿瘤大会ASCO、美国血液年会ASH、欧洲血液年会EHA、日本血液年会JSH)汇报团队免疫治疗的最新进展。

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#cart #carttherapy #CAR_T #leukemia #lymphoma #cancer #tumor #bloodcancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
BCMA CAR-T Therapy Paves New Path for Treating High-Risk Multiple Myeloma
**BCMA CAR-T Therapy Paves New Path for Treating High-Risk Multiple Myeloma**

Multiple Myeloma
Multiple Myeloma (MM) is a malignant tumor originating from plasma cells, known for its complex biology and treatment resistance, presenting significant challenges to patients’ survival. For high-risk MM, especially in cases of relapse and refractory disease after multiple lines of therapy, treatment options become critically important. Recently, a team led by Professor Qian from a Chinese Hospital successfully treated a high-risk relapsed/refractory MM (RRMM) patient using BCMA CAR-T cell therapy, offering new hope for difficult-to-treat cases.
**Patient Overview**
The patient, a 56-year-old woman, initially sought treatment for persistent anemia and back pain. Baseline examinations revealed significant abnormalities, including a hemoglobin level of 67g/L, thrombocytopenia, and abnormal bone marrow findings. She was diagnosed with IgG-kappa type MM at stage ISS III/R-ISS III, with cytogenetic abnormalities and high-risk factors such as t(14;16) and 1q21 amplification. The disease rapidly progressed despite multiple lines of therapy, including VRd and DVD regimens, with external manifestations of extramedullary disease (EMD), indicating a poor prognosis.
**Treatment Journey**
The patient initially underwent VRd induction therapy, followed by DVD treatment when the disease progressed. Despite efforts, she developed multiple extramedullary lesions, leading to the use of the Dara+DECP regimen and autologous stem cell transplantation (ASCT). While these treatments provided partial remission, the disease relapsed within months, with extramedullary involvement further complicating the prognosis.
Given the poor prognosis and lack of effective treatments for EMD in MM, the decision was made to pursue BCMA CAR-T cell therapy, specifically with **Equecabtagene Autoleucel**, China’s first CAR-T product for treating MM. This therapy has demonstrated an impressive overall response rate (ORR) of 100% in patients with extramedullary disease, with a complete remission (CR) rate of 78.6%.
**CAR-T Therapy and Outcomes**
Following preconditioning with FC regimen, the patient underwent BCMA CAR-T therapy. After CAR-T infusion, the patient experienced mild cytokine release syndrome (CRS), which was successfully managed with supportive care. Over the course of three months, significant clinical improvements were observed. PET-CT scans showed no residual disease, and bone marrow biopsies were negative for clonal plasma cells. The patient achieved stringent complete remission (sCR).
Eight months after CAR-T therapy, follow-up results continue to show no evidence of disease, with the patient maintaining CR. This case highlights the long-lasting anti-tumor effects of Equecabtagene Autoleucel, a fully human BCMA CAR-T product, which offers low immunogenicity and sustained CAR-T cell persistence in vivo.
**Implications for Future Treatment**
This success story offers new hope for patients with high-risk and refractory MM, especially those with extramedullary involvement. It also provides valuable clinical insights into the use of BCMA CAR-T therapy as a promising treatment strategy. The case demonstrates that for patients with high-risk MM, comprehensive risk assessment considering genetic characteristics, treatment responses, and future treatment plans is essential for personalized care.
In conclusion, the application of Equecabtagene Autoleucel CAR-T therapy represents a significant breakthrough in the treatment of high-risk, refractory MM, particularly in cases involving extramedullary disease. This innovative approach not only extends survival but also enhances the quality of life for patients who have exhausted other treatment options, marking a new chapter in the fight against multiple myeloma.
🎉🎉To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CAR_Therapy #BCMA #CancerTreatment #InnovativeMedicine #Immunotherapy #ExtramedullaryDisease #RelapsedMM #ChinaHealthcare #GlobalHealth
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s Fully Human BCMA CAR-T Therapy: Bringing New Hope for High-Risk Relapsed/Refractory Multiple Myeloma
China’s Fully Human BCMA CAR-T Therapy: Bringing New Hope for High-Risk Relapsed/Refractory Multiple Myeloma

Mutiple Myeloma
Multiple Myeloma (MM) is a complex and aggressive cancer originating from plasma cells, posing significant treatment challenges due to its resistance to therapy. For patients with high-risk MM, especially those who experience relapse after multiple lines of treatment, finding an effective therapy is critical. Recently, Chinese medical team successfully treated a case of high-risk relapsed/refractory MM (RRMM) using fully human BCMA CAR-T cell therapy, offering new hope for such difficult cases.
**Case Overview**
The patient, a 56-year-old woman, presented with anemia during a routine check-up and was later diagnosed with MM. Despite undergoing several treatment regimens, including VRd (bortezomib, lenalidomide, dexamethasone) and Dara-DECP followed by autologous stem cell transplantation (ASCT), the disease continued to progress, with extramedullary disease (EMD) manifestations complicating the case.
**Challenges and Treatment Journey**
After initial treatments failed to achieve long-term remission, the patient’s condition worsened, with new lesions detected in the pancreas and multiple subcutaneous nodules indicating possible metastasis. Given the aggressive nature of the disease and the presence of EMD, which is associated with a poor prognosis, the medical team opted for a BCMA-targeted CAR-T cell therapy using Iquarense (Ikaros CAR-T), the first CAR-T product approved for MM treatment in China.
**CAR-T Therapy and Results**
Following pre-conditioning with a fludarabine-cyclophosphamide (FC) regimen, the patient received the BCMA CAR-T therapy. The treatment was well-tolerated, with only mild cytokine release syndrome (CRS) observed. Remarkably, within three months post-treatment, PET-CT scans showed no signs of active disease, and the patient achieved a complete response (CR), which has been sustained for eight months.
**Significance and Future Implications**
This case highlights the potential of BCMA CAR-T therapy as a powerful option for patients with high-risk, relapsed/refractory MM, particularly those with EMD. The successful outcome not only provides new hope for patients facing similar challenges but also contributes valuable insights for future treatment strategies. Iquarense, with its low immunogenicity and prolonged persistence in the body, represents a promising advance in the fight against this formidable disease.
For patients with high-risk MM, it is crucial to consider genetic factors, treatment response, and overall disease dynamics when selecting a therapeutic approach. As this case demonstrates, BCMA CAR-T therapy offers a viable path forward, particularly for those with limited options due to disease progression or EMD.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #HopeForCancerPatients
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Groundbreaking Results for Chinese CAR-T Therapy: 86% Response Rate of BCMA/GPRC5D Dual-Target Treatment Multiple myeloma
**Groundbreaking Results for Chinese CAR-T Therapy: 86% Response Rate of BCMA/GPRC5D Dual-Target Treatment**

Multiple myeloma
Multiple myeloma (MM) is a challenging blood cancer, particularly in relapsed/refractory (R/R) cases. While therapies like proteasome inhibitors, immunomodulators, CD38 monoclonal antibodies, and stem cell transplants have improved outcomes for newly diagnosed patients, treatment-resistant forms of MM remain a serious concern. A new therapeutic approach combining two targets, BCMA and GPRC5D, may provide a breakthrough solution.
BCMA has long been a key target for treating R/R MM due to its expression in malignant plasma cells. However, BCMA-targeted therapies face limitations, including tumor cells losing or downregulating BCMA, leading to disease recurrence. This is where GPRC5D, a protein highly expressed in MM cells and linked to poor prognosis, comes in. By targeting both BCMA and GPRC5D, researchers aim to overcome the limitations of single-target therapies.
A collaboration between a leading Chinese hospital and research institutions has now delivered promising results. A Phase I clinical trial involving 21 patients with R/R MM using the dual-target BCMA/GPRC5D CAR-T cell therapy reported an overall response rate (ORR) of 86%, with 75% of patients achieving a complete response (CR). Notably, even patients whose cancer cells lacked BCMA or GPRC5D expression showed significant improvement, underscoring the versatility of this treatment.
**Key Results from the Study:**
– 21 patients with advanced, heavily pre-treated R/R MM participated.
– Of the 12 patients who received the optimal dosage of 2.0×10⁶ CAR-T cells/kg, 86% showed clinical response, with 75% achieving complete remission.
– Importantly, patients with BCMA or GPRC5D-negative cancer cells also responded well to treatment.
– The treatment was well-tolerated, with manageable side effects, including 71% of patients experiencing mild to moderate cytokine release syndrome (CRS).
This trial, published in *The Lancet Haematology*, represents a major step forward in MM treatment. The dual-target approach addresses the limitations of BCMA-targeted therapies, offering new hope to patients who have exhausted conventional options.
Looking ahead, this innovative therapy could reshape the future of multiple myeloma treatment, offering a powerful new tool in the fight against this complex disease.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerResearch #MultipleMyeloma #CAR_TTherapy #MedicalInnovation #OncologyBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma
### Overcoming Challenges and Embracing New Life! – Breakthrough Progress of Eque-cel in the Treatment of Refractory Multiple Myeloma

Multiple Myeloma
In the treatment of multiple myeloma (MM), how do we find new breakthroughs for patients who have not achieved complete remission (CR) after multiple rounds of chemotherapy? Research by Chinese medical professors has provided an exciting answer: Eque-cel (BCMA CAR-T therapy).
**Patient Background:**
This 58-year-old female patient was initially admitted to the hospital due to numbness and pain in both lower limbs and was eventually diagnosed with multiple myeloma. Despite receiving various treatment regimens, including VRD and SVPD, the results were unsatisfactory, and complete remission was not achieved. Faced with refractory characteristics, the doctors decided to try a more innovative treatment plan—CAR-T cell therapy.
**Treatment Process:**
In September 2023, the patient began peripheral blood mononuclear cell collection, followed by bridging therapy, and in November 2023, she received the Eque-cel infusion. Remarkably, just one month later, the patient achieved hematologic complete remission (CR) with minimal residual disease (MRD) negativity. After six months of follow-up, the patient maintained this excellent therapeutic effect.
**Professor’s Insights:**
Chinese medical professors pointed out that the advent of Eque-cel has brought new hope to refractory MM patients. The drug demonstrated significant efficacy in the FUMANBA-1 study: the overall response rate was as high as 98.9%, with 82.4% achieving complete remission, and 97.8% of patients achieving MRD negativity. The 12-month sustained MRD negativity rate reached 81.7%, and the PFS rate was 85.5%.
This outstanding result proves the significant advantage of Eque-cel in improving the depth of remission for MM patients, bringing hope for long-term survival to many refractory patients.
**Future Outlook:**
As the application and research of Eque-cel continue, we look forward to it providing better treatment options and survival opportunities for more MM patients. This new treatment plan is bringing a ray of hope to this stubborn disease and providing valuable experience for clinical experts worldwide.
**Stay Tuned:**
We will continue to follow the latest developments and research progress of Eque-cel, looking forward to its greater role globally, bringing hope and blessings to more patients.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CARTTherapy #MedicalBreakthrough #EqueCel #CancerTreatment #MedicalResearch #Hematology #PatientCare #OncologyInnovation #HopeForPatients #BCMA #CART #MM #RRMM
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook
**Chinese Medical Team: Long Survival and Significant Benefits with BCMA CAR-T Treatment for RRMM: A 5-Year Outlook**

RRMM
Chimeric antigen receptor (CAR) T-cell therapy is one of the most promising new treatments for relapsed/refractory multiple myeloma (RRMM), but reports on its long-term efficacy and safety are limited. As early as 2022, Professor Du Juan’s team from the Department of Hematology at Shanghai Changzheng Hospital published a Phase I/II study demonstrating that patients with poor physical status could also benefit from CAR-T therapy. Recently, the team updated their findings with a five-year long-term follow-up, focusing on factors affecting long-term clinical benefits. The results were published in *Clinical Cancer Research*. The following summary of the study’s content is provided by *Cancer Information* for readers’ benefit.
### Evidence for Long-Term Efficacy and Safety of BCMA CAR-T Cell Therapy
#### Patient Characteristics
The study included 49 RRMM patients who had all received at least three prior lines of therapy before undergoing BCMA CAR-T cell treatment. At enrollment, 20 patients (40.82%) had poor physical status (ECOG performance status of 3-4), 42.86% had high-risk cytogenetic features, and 63.27% had received four or more lines of treatment. At the time of infusion, 79.59% had progressive disease. Among the patients with poor physical status, 30% had extramedullary disease (EMD), 45% had high-risk cytogenetic features, 70% had received four or more lines of treatment, and 80% had progressive disease after their last line of treatment.
#### Efficacy Evaluation of BCMA CAR-T Cell Therapy HDS269B
After a median follow-up of 59.0 months, the study showed an overall response rate (ORR) of 77.55%. The ORR was similar across patients with different ECOG scores. The median progression-free survival (PFS) was 9.5 months, and the median overall survival (OS) was 20.0 months. The five-year PFS and OS rates were 21.3% and 31.4%, respectively. For patients with ECOG scores of 0-2, the median PFS was 11.0 months, compared to 4.0 months for those with scores of 3-4 (P=0.18). The median OS was 41.8 months for ECOG 0-2 patients and 10.5 months for ECOG 3-4 patients (P=0.015).
Patients who had previously undergone four or more lines of therapy had significantly shorter PFS and OS compared to those who had received fewer than four lines (PFS: P=0.012; OS: P=0.0049). Among the 11 patients with EMD at enrollment, the ORR was 64% for those with EMD and 82% for those without EMD. However, median PFS and OS were notably shorter in patients with EMD (PFS: 3.0 months vs. 10.5 months, P=0.06; OS: 5.0 months vs. 24.0 months, P=0.03).
#### MRD-Negative Status and CAR-T Cell Persistence Indicate Better Long-Term Survival
Minimal residual disease (MRD) negativity was significantly associated with longer PFS and OS. In this study, MRD data were available for 22 patients on day 28 post-infusion, with 14 patients (63.64%) achieving MRD negativity (10^-4). These patients experienced significantly longer PFS and OS compared to MRD-positive patients. Similar associations were observed with MRD status at 3 and 6 months post-infusion.
The expansion of CAR-T cells was also closely related to clinical outcomes. Patients who achieved partial response (PR) or better had higher CAR-T cell peak levels. Patients without disease progression five years post-infusion had significantly higher CAR-T cell expansion peaks than those with progression. Additionally, the duration of CAR-T cell persistence correlated with longer PFS and OS, with patients having CAR-T cells persisting for ≥6 months, ≥12 months, ≥24 months, and ≥36 months showing significantly better PFS and OS than those without detectable CAR-T cells.
#### Controlled Safety Profile of BCMA CAR-T Cell Therapy HDS269B
No new CAR-T cell-related toxicities were observed during long-term follow-up. All patients experienced at least one adverse event (AE), with the most common long-term (≥28 days post-infusion) grade ≥3 AEs being hematologic in nature. No second primary malignancies or delayed immune effector cell-associated neurotoxicity syndrome (ICANS) were observed.
This study also included survival analysis, classifying patients by PFS and OS. The results indicated that ECOG 0-2 status, fewer than four prior therapies, and CAR-T cell persistence for ≥6 months were independently associated with longer survival.
### The Potential of BCMA CAR-T Therapy and the Need for Future Optimization
Through a five-year long-term follow-up of 49 RRMM patients, this study systematically evaluated the efficacy and safety of BCMA CAR-T cell therapy HDS269B. The findings suggest that poor physical status is not a contraindication for CAR-T therapy, thus broadening the indications for this treatment. While the results are encouraging, the study has some limitations, including its open-label, single-arm design and small sample size, which, combined with the long follow-up period, could lead to some patient attrition. Furthermore, despite the lack of new severe toxicities, long-term safety requires continued observation.
Overall, this study underscores the importance of BCMA CAR-T cell therapy in the treatment of RRMM and provides a crucial basis for exploring and applying CAR-T immunotherapy in the frontline treatment of multiple myeloma.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +86137 1795 9070
Email: doctor.huang@globecancer.com
#CAR_Therapy #BCMACART #MultipleMyeloma #CancerResearch #LongTermSurvival #MedicalInnovation #Hematology #CancerTreatment #Immunotherapy #OncologyAdvances
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

2024 EBMT : China’s First RRMM CAR-T Therapy Equecabtagene Autoleucel: Efficacy Unaffected by Patients’ Baseline sBCMA Plasma Levels
2024 EBMT : China’s First RRMM CAR-T Therapy Equecabtagene Autoleucel: Efficacy Unaffected by Patients’ Baseline sBCMA Plasma Levels

RRMM
In recent years, CAR-T cell therapy targeting BCMA has emerged as a groundbreaking treatment for multiple myeloma, offering new hope to patients. At the 50th European Society for Blood and Marrow Transplantation (EBMT) Annual Meeting, held from April 14-17, 2024, in Glasgow, the team led by Professor Qiu Lugui presented the latest subgroup analysis results from the FUMANBA-1 study (Abstract OS10-04) on China’s first BCMA-targeted CAR-T therapy, Iquilencel (CT103A).
BCMA (B-cell maturation antigen) is a promising therapeutic target for multiple myeloma (MM), with soluble BCMA (sBCMA) levels in the blood reflecting tumor burden. High sBCMA levels can interfere with the effectiveness of BCMA-targeted therapies, including CAR-T, by competing with cell-surface BCMA for binding, which can lead to reduced efficacy. In contrast, Iquilencel has been designed to minimize the impact of sBCMA on treatment outcomes through careful selection of its single-chain variable fragment (scFv).
The FUMANBA-1 phase II study (NCT05066646) in Chinese patients with relapsed/refractory multiple myeloma (RRMM) has demonstrated that Iquilencel can induce deep and durable responses, with a complete response (CR) rate of 82.4% and a 12-month progression-free survival (PFS) rate of 85.5%. This study aimed to explore whether baseline serum sBCMA levels affect clinical outcomes following Iquilencel infusion.
### Study Methods and Results
The study used enzyme-linked immunosorbent assay (ELISA) to measure serum sBCMA levels and digital droplet PCR (ddPCR) to monitor CAR transgene copy numbers in patients’ peripheral blood. Baseline serum sBCMA levels were classified into high (≥225.1 ng/mL) and low (<225.1 ng/mL) groups. Results showed that high sBCMA levels were significantly associated with high tumor burden, advanced R-ISS and DS stages, and high BCMA expression. However, there were no significant differences in CAR-T cell expansion, AUC (Area Under the Curve) during the first 28 days, or cell persistence between the high and low sBCMA groups.
Patients with high baseline sBCMA levels had overall response rates (ORR) and ≥CR rates of 100% and 80%, respectively, compared to 97.8% and 84% in the low sBCMA group. Analysis showed no significant correlation between baseline characteristics (including sBCMA levels) and CR/sCR achievement. Additionally, there were no significant differences in minimal residual disease (MRD) negativity rates, 18-month sustained MRD negativity rates, PFS, and overall survival (OS) between the two groups.
### Conclusion
The findings from the FUMANBA-1 study indicate that Iquilencel’s efficacy is not influenced by baseline sBCMA levels, making it a universally applicable and promising treatment option for RRMM patients. Its unique fast-dissociation and low-exhaustion properties, similar to those of healthy T-cell receptors, enable Iquilencel to remain effective and persistent in patients’ bodies regardless of sBCMA levels.
Professor Qiu Lugui from the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences, and Professor Li Chunrui from Tongji Hospital, Huazhong University of Science and Technology, noted, “sBCMA is an important biomarker of tumor burden in multiple myeloma and a key factor influencing prognosis. Accumulation of sBCMA can inhibit the function of BCMA CAR-T cells. However, our study shows that Iquilencel can overcome the challenges posed by high baseline sBCMA levels, providing significant and lasting responses for RRMM patients.”
These results underscore Iquilencel as an ideal treatment choice for RRMM, offering hope for more effective and long-lasting therapeutic outcomes.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#EBMT2024 #CAR_T #MultipleMyeloma #Iquilencel #EquecabtageneAutoleucel #sBCMA #CancerResearch #Immunotherapy #MedicalBreakthrough #Biopharmaceuticals
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient
22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient

Patient story
Subtitle: Fighting for Love! A story of miraculous rebirth after all treatment options failed for a late-stage multiple myeloma patient in Thailand, who underwent CAR-T therapy in China.
Preface: When all treatment options had been exhausted, cancer progressed rapidly, and doctors regretfully said that Ms. M had no response to any medication. This situation could only lead to palliative care, meaning Ms. M had no chance to fight this disease anymore; her life was now on borrowed time. Fortunately, Ms. M’s family found new hope in China – CAR-T therapy. With the companionship of her family, Ms. M came to China and underwent the world’s first fully human BCMA CAR-T therapy. In just 22 days after treatment, all tumors miraculously disappeared, achieving complete remission (CR), and Ms. M was given a new lease on life!
In June 2021, the life of 58-year-old Ms. M experienced persistent back pain for over months. After detailed examinations at the hospital,resulting in a diagnosis of multiple myeloma. To quickly control the condition, Ms. M received treatments including PCD, DVD, bortezomib and lenalidomide, autologous hematopoietic stem cell transplantation.
In June 2023, the disease relapsed and Ms. M did not respond to any of these treatments. Local doctors informed Ms. M’s family that apart from palliative care, they were powerless, indicating that Ms. M’s life was now on borrowed time! Just as Ms. M was in dire straits, on June 30, 2023, the world’s first fully human BCMA CAR-T therapy – Equecabtagene Autoleucel, was shockingly launched in China, becoming a lifesaving straw for her. Ms. M and her family decided to seek treatment in China.
In September 2023, Professor Li Ping’s team at Shanghai Tongji Hospital developed a personalized Equecabtagene Autoleucel(BCMA CAR-T) therapy plan for Ms. M.
On September 8, 2023, Ms. M finally officially entered the CAR-T treatment process. After all examination items met the requirements, single-cell collection was performed first.
Bridge therapy began on September 11, 2023;
Local radiotherapy to relieve bone pain started on September 24, 2023;
Fludarabine plus cyclophosphamide chemotherapy was administered from October 9-12, 2023;
Finally, on October 14, a small bag of milky-white liquid was infused into Ms. M’s body. The doctor said that inside were billions of “special” T cells that could precisely kill multiple myeloma cells. Once inside the body, they would initiate a mode of frenzied sweeping, wiping out cancer cells completely.
What shocked everyone was that the examination results 22 days after CAR-T treatment showed that Ms. M had achieved hematologic CR, meaning that no cancer cells were detected in her blood. Her pain and anemia were also reversed. It was simply a miracle!
Every day now is precious to Ms. M. She can go shopping, chat with her family, enjoy delicious food with her family, and travel around the world with them, returning to the happy times before she fell ill. The only difference is that Ms. M and her family visit a fixed destination every month now – Shanghai, China. They have become accustomed to hearing the sacred announcement from the doctors here: no cancer in her body, continuing remission.
This is a microcosm of countless cancer patients overcoming the disease. The emergence of CAR-T cell therapy has brought new hope to cancer patients. I believe that in the future, more patients will be able to regain a new life like Ms. M.
Reference:
[1]Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.
[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient
22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient

Patient story
In July 2017, 58-year-old Ms. M was diagnosed with multiple myeloma. After receiving treatments at the best hospital in Thailand, including PCD, DVD, bortezomib and lenalidomide, autologous hematopoietic stem cell transplantation. The disease rapidly relapsed. Local doctors informed Ms. M’s family that apart from palliative care, they were powerless.
Just as Ms. M was in dire straits, on June 30, 2023, the world’s first fully human BCMA CAR-T therapy – Equecabtagene Autoleucel, was shockingly launched in China, becoming a lifesaving straw for her. Ms. M and her family decided to seek treatment in China.
In September 2023, Professor Li Ping’s team at Shanghai Tongji Hospital developed a personalized Equecabtagene Autoleucel(BCMA CAR-T) therapy plan for Ms. M. What shocked everyone was that the examination results 22 days after CAR-T treatment showed that Ms. M had achieved hematologic CR, meaning that no cancer cells were detected in her blood. Her pain and anemia were also reversed. It was simply a miracle!
Reference:
[1]Yuting Yan, et al. Blood Adv. 2019 Oct 8;3(19):2895-2904.
[2] 2023 IMS. P-290.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

#EBMT Conference Reveals the Best CAR-T Therapy for Multiple Myeloma – Equecabtagene Autoleucel
#EBMT Conference Reveals the Best CAR-T Therapy for Multiple Myeloma – Equecabtagene Autoleucel

Multiple myeloma
