Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

The significance of the 11 CD19 and BCMA CAR-T therapies currently approvedin China and the U.S. forpatients with hematologic malignancies.
The significance of the 11 CD19 and BCMA CAR-T therapies currently approvedin China and the U.S. forpatients with hematologic malignancies.
目前中美上市的11款CD19和BCMA CAR-T对血液瘤患者的意义
Expert:
**Jing Pan**
**Associate Chief Physician**
**Department of Pediatric Hematology, Beijing Gaobo Hospital**
**Professional Memberships:**
– Member, Pediatric Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Hematologic Oncology Professional Committee of the Chinese Anti-Cancer Association
– Youth Committee Member, Clinical Application Professional Committee of the Chinese Medical Biotechnology Association
**Expertise:**
Dr. Pan currently manages an 80-bed pediatric hematology unit and has extensive experience in pediatric hematology, particularly in CAR-T cell immunotherapy, with nearly 10 years of experience in the field. She is dedicated to stratified CAR-T treatment, optimizing the management of complications during CAR-T therapy, and establishing an efficacy monitoring system post-treatment. Dr. Pan and her team have accumulated one of the largest single-center case collections globally, particularly in the areas of sequential CAR-T therapy for improving long-term outcomes in B-ALL and in exploring autologous and allogeneic CD5, CD7 CAR-T therapy for T-ALL/LBL.
Her related clinical research on CD7 CAR-T, CD19 CAR-T, CD22 CAR-T, and CD19-22 sequential CAR-T has been published in leading international journals such as *Lancet Oncology*, *JCO*, *Blood*, *JHO*, and *Leukemia*. Additionally, she has frequently presented the latest advancements in her team’s immunotherapy research at both domestic and international conferences, including ASCO, ASH, EHA, and JSH.

 To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#cart #carttherapy #CAR_T #leukemia #lymphoma #cancer #tumor #bloodcancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

A Miraculous Journey: Israeli Artist Finds Cure for Multiple Myeloma in Hangzhou China






Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Clinical Breakthrough: Chinese CAR-T – Inaticabtagene Autoleucel Revolutionizing Hematologic Cancer Therapy


Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exploring Tumor Vitality: Chinese CAR-T Therapy Grants Patients Complete Remission


Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Two Years of Complete Remission(Lymphoma), CAR-T therapy has given her a new lease on life.
“Two Years of Complete Remission, CAR-T therapy has given her a new lease on life. She Thought the Journey Was Over”

Yun is a 78-year-old patient who achieved a continuous complete remission for two and a half years despite relapsed/refractory diffuse large B-cell lymphoma. Diagnosed in 2019, after enduring six rounds of chemotherapy, she initially achieved complete remission as confirmed by her healthcare team. Unfortunately, the period of remission was short-lived as Yun soon experienced disease relapse, and subsequent second-line treatments failed to yield positive results.
“I vividly remember when Director Wang Li encouraged me, mentioning an advanced method called Car-T, but it was quite expensive. I was hesitant, but I discussed it with my son when I got home. When my son and daughter-in-law heard about it, they insisted on treatment. My son said, ‘Mom, you’re the only mother I have in this world. As long as you’re here, our home is complete. Money can be earned again, and if the treatment isn’t successful, at least we won’t have regrets.’ I was deeply moved. At my age, I’ve already shown strength through previous treatments. I believe that wherever my health takes me is where I belong. Everyone’s support gave me a reason to stay strong again.”
In 2021, CAR-T cell therapy was approved and launched in China, and Yun underwent this treatment at Ruijin Hospital.
Dr. Wang Li, Director of Hematology at Ruijin Hospital, explained, “CAR-T cell therapy provides new treatment options for a wide range of cancer patients.”
“Before planning Yun’s CAR-T cell therapy, lymphoma experts, considering her current tumor status and medical history, anticipated potential adverse reactions during the treatment process and discussed handling protocols. Yun’s entire journey with CAR-T cell therapy was challenging. The medical team meticulously coordinated their efforts, successfully addressing adverse reactions after CAR-T treatment, ultimately averting potential risks.”
Despite lying in her hospital bed, Yun deeply appreciates the relentless dedication of her medical caregivers. “Sometimes, directors come to see me after 10 p.m., telling me they worked late but had to check on me; some arrive by my bedside as early as 7 a.m., concerned about my well-being. I continuously remind myself that I must strive, persist, and live up to the efforts made by doctors and my entire family,” said the 78-year-old Yun . “I made it through.”
#CARTCellSuccess #CancerWarrior #CompleteRemissionChronicles #FamilySupportMatters #CAR-TBreakthrough #InspiringHealthJourney #MedicalMiracles #NeverTooLateToFight #Cancerfight #Bloodcancer
#lymphoma
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Breakthrough Advances in CAR-T Cell Therapy Combined with TKI for Malignant Hematologic System Tumors



Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
Chinese Research: Hopes of CAR-T in Second-Line Treatment of LBCL(Lymphoma)


Breakthrough medical research in China brings hope for multiple myeloma patients.
Eque-cel brings new treatment prospects for multiple myeloma.
From December 9th to 12th, the Annual American Society of Hematology Meeting (ASH 2023) was held in San Diego, USA. At this conference, the FUMANBA-1 study, led by Professors Chunrui Li from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Lugui Qiu from the Institute of Hematology, Chinese Academy of Medical Sciences, conducted at 14 medical centers nationwide, demonstrated the efficacy and characteristics of sustained minimal residual disease (MRD) negativity in multiple myeloma (MM) patients treated with Eque-cel injection (CT103A). Their achievements were included in the oral presentation at the conference.

Current MM Treatment and Challenges of CAR-T Therapy CAR-T cell therapy holds significant importance in the treatment of multiple myeloma, overcoming patients’ immunodeficiency and tolerance issues. Currently, China has approved three CAR-T cell drugs targeting multiple myeloma. Compared to traditional treatments, CAR-T cell therapy enhances immune function without relying on the major histocompatibility complex (MHC), improving the activity of NK cells, T cells, and B cells while effectively activating T cells. However, despite significant advancements in CAR-T cell therapy in the MM field, some patients still struggle to maintain treatment effectiveness, leading to potential relapses, with antigen escape being one of the important mechanisms causing relapse.
Furthermore, the safety of CAR-T therapy is a major concern, including adverse reactions such as cytokine release syndrome (CRS), neurotoxicity, infections, B cell depletion, and hypogammaglobulinemia. Hence, monitoring patient indicators throughout the entire CAR-T treatment process, from pre-treatment to infusion, is crucial for timely intervention in adverse reactions.
100% MRD Negativity Rate for CR and Above Patients! Eque-cel Injection Brings Redemption for Multiple Myeloma
Eque-cel injection (CT103A) is a fully humanized CAR-T cell therapy drug targeting B cell maturation antigen (BCMA), approved by the National Medical Products Administration (NMPA) for adult refractory/relapsed multiple myeloma patients in China. In the Phase II FUMANBA-1 study, this treatment showed significant and sustained efficacy.
As of December 31, 2022, with a median follow-up time of 18.07 months, deep and sustained remissions were observed in 103 evaluable patients. The overall response rate (ORR) among these 103 patients was 96.1%, with a strict complete response (sCR)/complete response (CR) rate of 77.7%. Among 91 subjects with no previous CAR-T treatment history, the ORR was 98.9%, and the sCR/CR rate reached 82.4%, with a 12-month progression-free survival (PFS) rate of 85.5%.
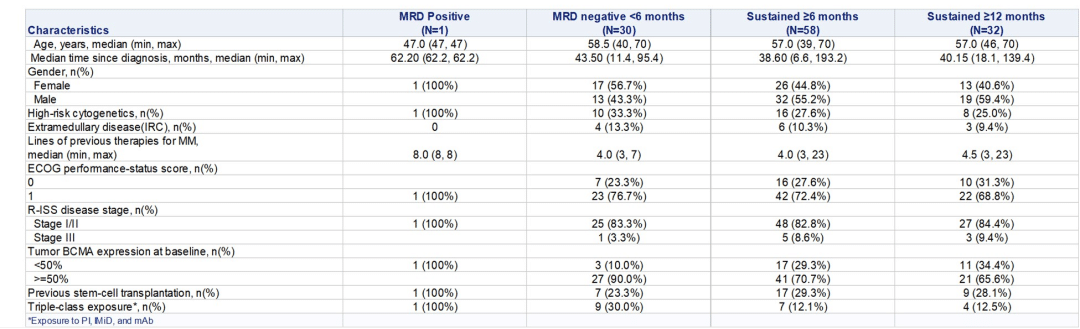
The minimal residual disease (MRD) negativity rate for the entire population was 94.2%, reaching 100% for CR and above patients. The median time to MRD negativity was 15 days, and 80.8% of patients maintained MRD negativity 12 months post-infusion. Moreover, the Eque-cel injection exhibited a relatively long median persistence in the body, lasting up to 307.5 days.
According to Professor Chunrui Li, “The sustained MRD-negative status is closely related to the improvement in PFS of patients receiving Eque-cel treatment.”
Professor Chunrui Li’s team studied the characteristics of patients maintaining MRD negativity for ≥6 months and ≥12 months in the FUMANBA-1 study. The research revealed that among 88 patients achieving MRD negativity, 78.4% maintained MRD negativity for at least 6 months, and 74.4% sustained MRD negativity for at least 12 months. Patients with sustained MRD negativity showed longer…

Professor Chunrui Li:
Chief Physician, Doctoral Supervisor
Secretary of the Hematology Department Party Committee, Deputy Director of Medicine Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Professional
Focus: Immunotherapy for Hematological Malignancies
Member of the 11th Committee of the Plasma Cell Disease Professional Group of the Hematology Branch of the Chinese Medical Association
Chairman of the Expert Committee on Hematology (Hubei), Geriatrics Branch, Chinese Geriatrics and Gerontology Society
Standing Committee Member of the Geriatric Medicine Branch, Chinese Geriatrics and Gerontology Society
Youth Committee Member of the Oncology Branch, Chinese Anti-Cancer Association Committee Member of the Myeloma and Plasma Cell Disease Professional Group, 5th Committee of the Chinese Society of Clinical Oncology (CSCO) Leukemia Alliance & Lymphoma Alliance
Vice Chairman of the Blood Branch of the Hubei Medical Immunology Association Principal Investigator of four National Natural Science Foundation projects; published more than 20 SCI papers as first author or corresponding author, including Blood.
#EqueCelTreatment #MMResearch #CARTTherapy #MRDNegativity #CancerTreatment #MedicalBreakthrough #FUMANBA1Study #Immunotherapy #CancerResearchUpdate #CancerResearch #ASH2023 #BloodCancer #EqueCel #MRD #MultipleMyeloma
