Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Approval of #Liver Cancer CAR-T Product Targeting #GPC3 Propels China’s CAR-T Therapy Forward
 Approval of #Liver Cancer CAR-T Product Targeting #GPC3 Propels China’s CAR-T Therapy Forward
Approval of #Liver Cancer CAR-T Product Targeting #GPC3 Propels China’s CAR-T Therapy Forward

Liver cancer, CAR-T
CT011
 On January 15, 2024, #CARsgen Pharmaceuticals announced that its autologous #CARTcell candidate product #CT011, targeting Glypican-3 (GPC3), has been approved for clinical use in China. It is intended for the treatment of GPC3-positive stage IIIa hepatocellular carcinoma patients at risk of recurrence after surgical resection.
On January 15, 2024, #CARsgen Pharmaceuticals announced that its autologous #CARTcell candidate product #CT011, targeting Glypican-3 (GPC3), has been approved for clinical use in China. It is intended for the treatment of GPC3-positive stage IIIa hepatocellular carcinoma patients at risk of recurrence after surgical resection.
Glypican-3 (GPC3)
 Glypican-3 (GPC3)plays a crucial role in regulating cell growth and differentiation and is closely associated with the occurrence and progression of liver cancer. GPC3 is expressed in reproductive system tumors such as hepatocellular carcinoma, ovarian clear cell carcinoma, and yolk sac tumors. Its expression rate in hepatocellular carcinoma reaches 74.8%, while it is virtually absent in normal liver tissue, making it an ideal new target for #livercancer #CARTtherapy.
Glypican-3 (GPC3)plays a crucial role in regulating cell growth and differentiation and is closely associated with the occurrence and progression of liver cancer. GPC3 is expressed in reproductive system tumors such as hepatocellular carcinoma, ovarian clear cell carcinoma, and yolk sac tumors. Its expression rate in hepatocellular carcinoma reaches 74.8%, while it is virtually absent in normal liver tissue, making it an ideal new target for #livercancer #CARTtherapy.
Promising Target
 Due to its tumor specificity, GPC3 is considered a promising target for cancer immunotherapy. Higher expression of GPC3 in hepatocellular carcinoma is associated with poorer prognosis.
Due to its tumor specificity, GPC3 is considered a promising target for cancer immunotherapy. Higher expression of GPC3 in hepatocellular carcinoma is associated with poorer prognosis.
Frontiers in Immunology
 In August 2022, a Chinese medical team published a long-term survival case report of treating advanced hepatocellular carcinoma with CT011 in the journal “#FrontiersinImmunology.” The study reported complete remission (#CR) and long-term survival in a patient with advanced hepatocellular carcinoma after receiving GPC3 CAR-T cell therapy in combination with the multi-target kinase inhibitor sorafenib.
In August 2022, a Chinese medical team published a long-term survival case report of treating advanced hepatocellular carcinoma with CT011 in the journal “#FrontiersinImmunology.” The study reported complete remission (#CR) and long-term survival in a patient with advanced hepatocellular carcinoma after receiving GPC3 CAR-T cell therapy in combination with the multi-target kinase inhibitor sorafenib.
 Results showed good tolerance to CT011 combined with sorafenib treatment. The patient achieved partial remission (PR) from the third month onwards and attained complete remission at the 12th month after the first CT011 infusion. The tumor did not progress for over 36 months, maintaining complete remission status for over 24 months after the first infusion.
Results showed good tolerance to CT011 combined with sorafenib treatment. The patient achieved partial remission (PR) from the third month onwards and attained complete remission at the 12th month after the first CT011 infusion. The tumor did not progress for over 36 months, maintaining complete remission status for over 24 months after the first infusion.
Cancer Communications
 On October 12, 2023, CARsgen Pharmaceuticals announced the clinical efficacy of its independently developed CAR-T cell therapy targeting GPC3 in two patients with advanced hepatocellular carcinoma (HCC). The clinical trial results were published in “#CancerCommunications.” Both patients had inferior prognostic outcomes upon enrollment, with one having inferior vena cava tumor thrombus and the other having retroperitoneal lymph node metastasis.
On October 12, 2023, CARsgen Pharmaceuticals announced the clinical efficacy of its independently developed CAR-T cell therapy targeting GPC3 in two patients with advanced hepatocellular carcinoma (HCC). The clinical trial results were published in “#CancerCommunications.” Both patients had inferior prognostic outcomes upon enrollment, with one having inferior vena cava tumor thrombus and the other having retroperitoneal lymph node metastasis.
 In the clinical trial, one patient received local treatment for intrahepatic recurrent tumors and inferior vena cava tumor thrombus (microwave ablation and gamma knife) before receiving CAR-T cell therapy targeting GPC3. Six months later, AFP (alpha-fetoprotein) levels normalized, and imaging revealed no active tumors. The other patient underwent local treatment (microwave ablation and gamma knife) for controlling intrahepatic tumors, inferior vena cava tumor thrombus, and peritoneal lymph node metastases while receiving CAR-T cell therapy.
In the clinical trial, one patient received local treatment for intrahepatic recurrent tumors and inferior vena cava tumor thrombus (microwave ablation and gamma knife) before receiving CAR-T cell therapy targeting GPC3. Six months later, AFP (alpha-fetoprotein) levels normalized, and imaging revealed no active tumors. The other patient underwent local treatment (microwave ablation and gamma knife) for controlling intrahepatic tumors, inferior vena cava tumor thrombus, and peritoneal lymph node metastases while receiving CAR-T cell therapy.
 Encouragingly, both patients showed significant efficacy after combined local treatment and CAR-T cell infusion, maintaining tumor-free status during long-term follow-up, with both individuals being tumor-free for over 7 years. Throughout the follow-up period, both patients only received oral antiviral therapy for hepatitis B and did not undergo any other cancer treatment.
Encouragingly, both patients showed significant efficacy after combined local treatment and CAR-T cell infusion, maintaining tumor-free status during long-term follow-up, with both individuals being tumor-free for over 7 years. Throughout the follow-up period, both patients only received oral antiviral therapy for hepatitis B and did not undergo any other cancer treatment.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
 WhatsApp: +8613717959070
WhatsApp: +8613717959070
 Email: doctor.huang@globecancer.com
Email: doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

🌈Treatment Across Continents: American Myeloma Patient Finds New Life in China!🌈
🌈Treatment Across Continents: American Myeloma Patient Finds New Life in China!🌈

cancer fight
🌟Mr. C from California, USA
It’s yet another heartening piece of news as Mr. C from California, USA, undergoes cross-border treatment at Jiangsu Province People’s Hospital in China, successfully overcoming multiple myeloma and embracing a new lease on life! After meticulous treatment spanning one and a half months, Mr. C, accompanied by his loved ones, has made a successful recovery and been discharged from the hospital, becoming the first American patient to be successfully treated with CAR-T cell therapy for multiple myeloma in China.
🌞New treatment method
Mr. C, a 56-year-old American who is passionate about sports, discovered he had multiple myeloma after sustaining sports-related injuries. Despite enduring several unsuccessful treatment attempts, he refused to give up and began seeking treatment opportunities worldwide. However, the exorbitant cost of treating multiple myeloma in the USA deterred him. After comparing prices and data of CAR-T therapy for multiple myeloma between the USA and China, he ultimately decided to explore the new treatment method at Jiangsu Province People’s Hospital.
🌤Upon arriving in China,
Mr. C not only received meticulous care from medical staff but also had the opportunity to explore the scenic beauty of cities such as Nanjing, Suzhou, and Hangzhou, experiencing the unique cultural charm of China. During the CAR-T cell therapy treatment process, Mr. C underwent a life-threatening cytokine storm, but with the emergency intervention of the medical team, he successfully weathered the storm. Today, he has recovered and been discharged from the hospital, though slightly fatigued, he is still filled with hope and gratitude.
🔥Hope and Courage
Although Mr. C’s treatment has concluded, follow-up work will continue, with the medical team maintaining contact with his family doctor to closely monitor treatment effectiveness and subsequent conditions. As medical personnel, they sincerely hope that this treatment will provide Mr. C with more opportunities for survival.
☀️Mr. C also expressed his desire to revisit China, specifically Nanjing, to visit the experts and nurses who treated him, and of course, to continue savoring Chinese cuisine. This journey of treatment across continents not only granted Mr. C a new lease on life but also showcased the unity and dedication of medical personnel from both China and the USA. May this touching story inspire more people and bring forth more hope and courage!
⭐️CAR-T therapy
To assess whether the condition is suitable for CAR-T therapy, you can submit pathology reports, treatment history, and discharge summaries to the Medical Department of <Advanced Medicine in China> for preliminary evaluation!
You can send electronic copies or photos of genetic testing reports and diagnostic reports to:
💌: doctor.huang@globecancer.com
📞WhatsApp 137 1795 9070
✨The Medical Department will contact you as soon as receive the reports.
#CARTtherapy #LymphomaTreatment #CompleteRemission #MedicalAdvancements #CancerResearch #CARTProgress #HopeForPatients #SurvivingLymphoma #HealthcareInnovation #PatientStories #CancerCure #CARTSuccess #MedicalBreakthrough #ImprovingOutcomes #LymphomaAwareness #FightAgainstCancer #CARTJourney #MedicalMilestone #LongTermSurvival #BeatingCancer #lymphoma #cancerawareness
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

$160,000 per dose! The first approved CAR-T therapy in China, Zevor-cel by CARsgen, announces its initial price for the treatment of multiple myeloma.
🌈 $160,000 per dose! 🌈
⭐The first approved CAR-T therapy in China, Zevor-cel by CARsgen, announces its initial price for the treatment of multiple myeloma.

multiple myeloma
Zevor-cel
Cheapest CAR-T therapy
US CAR-T
Multiple Myeloma
In China
WhatsApp+8613717959070
doctor.huang@globecancer.com
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Putin Says Russia Is Close to Creating Cancer Vaccines. A Look at China’s Advancements
Putin Says Russia Is Close to Creating Cancer Vaccines. A Look at China’s Advancements

Putin

Cancer Vaccines
The personalized tumor neoantigen vaccine LK101 injection has been approved for clinical use!
Disease iNeo-Vac-P01 new antigen vaccine!
JCXH-211: Targeting multiple solid tumors with tumor neoantigen mRNA vaccine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
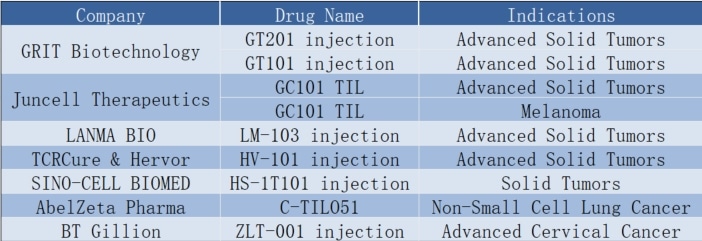
TIL Therapy: Revolutionizing Cancer Treatment Worldwide! China Accelerates into the Fast Lane!
🌟 **TIL Therapy: Revolutionizing Cancer Treatment Worldwide! 🌎** China Accelerates into the Fast Lane!
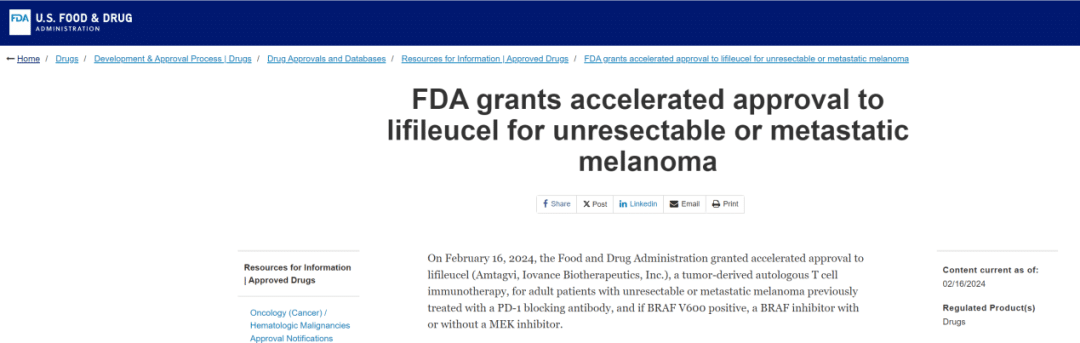
FDA

TIL therapy
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Hope For Patients of Gastric Cancer And Pancreatic Cancer – CT041 Typical Cases and Achievements

Gastric Cancer,
Pancreatic Cancer

Gastric Cancer,
Pancreatic Cancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Breaking News: Another Milestone for Domestic CAR-T Therapy! Clinical Benefit Rate Reaches 71.4% in CT041 Trials, Challenging Gastric and Pancreatic Cancers with Astonishing Results!

Gastric Cancer, Pancreatic Cancer
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Haematologica Spotlight:A Chinese team published the largest-scale pediatric-style regimen treatment for adult Ph-negative ALL
🔬 *Haematologica Spotlight* 🔬
A Chinese team published the largest-scale pediatric-style regimen treatment for adult Ph-negative ALL
Acute Lymphoblastic Leukemia (ALL)

Haematologica
Chinese Academy of Medical Sciences and Peking Union Medical College Hospital
The study, led by Dr. Wang Jianxiang and his team,
Patients achieving complete remission (CR)
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Exciting News Alert! China’s New TCR-T Product IND Approved for Synovial Sarcoma!
🌟 Exciting News Alert! China’s New TCR-T Product IND Approved for Synovial Sarcoma! 🌟

synovial sarcoma

Cell Rep Med
China’s first TCR-T product IND approval takes aim at synovial sarcoma.
TAEST16001,

TCR-T Therapy
How to Seek TCR-T Therapy Assistance
or click on the WhatsApp+8613717959070
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
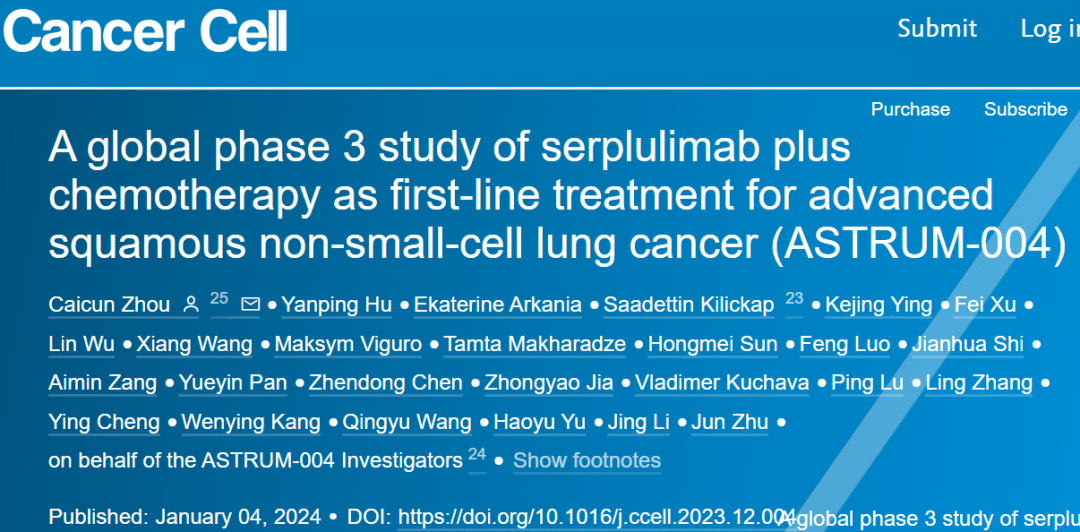
A New Era in Lung Cancer Treatment – Serplulimab Brings Innovative and Reliable Therapeutic Options!
**🌟 A New Era in Lung Cancer Treatment – Serplulimab Brings Innovative and Reliable Therapeutic Options! 🌟**

Cancer cell
📆CANCER CELL
🔬 Lung cancer
🌱NSCLC
🔍 ASTRUM-004
Serplulimab
🎉NEW ERA

👩🔬Lead
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Hengrui Pharmaceuticals ‘s indication for cervical cancer has been approved for clinical trials.
🌟 Chinese Hengrui Pharmaceuticals ‘s indication for cervical cancer has been approved for clinical trials.🌟

cervical cancer

Atezolizumab

The Lancet Oncology
🌸🎀Atezolizumab injection has been studied in various fields, including small cell lung cancer, #NSCLC non-small cell lung cancer, esophageal cancer, liver cancer, and cervical cancer. It has shown promising efficacy in small cell lung cancer. Based on the results of the SHR-1316-III-301 study, the application for the market approval of Atezolizumab injection combined with chemotherapy as first-line treatment for extensive-stage small cell lung cancer has been accepted and approved in China in March 2023. The study results have been published in the top international medical journal “The Lancet Oncology,” and the original research from China has been internationally recognized.

Hengrui Pharmaceutical
🚑In addition to Cervical cancer, we are currently urgently recruiting patients with B-cell lymphoma, T-cell lymphoma, T-cell leukemia (T-ALL), acute lymphoblastic leukemia, myeloma, and other types of cancer!
You can send electronic copies or photos of genetic testing reports and diagnostic reports to the 📩email address: doctor.huang@globecancer.com📩, or click on the ✉️WhatsApp+8613717959070✉️ icon on the homepage. The Medical Department will contact you as soon as they receive the reports.
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
Chinese Exciting Good News Update in Lung Cancer Treatment! ORR: 78.6%
 Meet Sunvozertinib, the game-changer in advanced lung cancer therapy!
Meet Sunvozertinib, the game-changer in advanced lung cancer therapy! 

Sunvozertinib, NSCLC

Lung Cancer
