Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
The CAR-γδT cell therapy QH104, independently developed by a Chinese team, has demonstrated a 100% disease control rate in patients with relapsed glioblastoma
**The CAR-γδT cell therapy QH104, independently developed by a Chinese team, has demonstrated a 100% disease control rate in patients with relapsed glioblastoma.**
#glioblastoma #CARγδT #QH104 #γδT #B7H3 #rGBM #CARgammaT

glioblastoma
A groundbreaking CAR-γδT cell therapy, QH104, developed by a Chinese team, is gaining attention in the global medical community for its potential in treating relapsed glioblastoma (rGBM). Targeting B7H3, QH104 is an allogeneic, off-the-shelf therapy with the promise of higher accessibility due to lower production costs compared to autologous cell therapies.
One of the unique strengths of γδT cells is their ability to recognize antigens without MHC restriction, reducing the risk of graft-versus-host disease (GvHD) and making them an ideal candidate for universal cell therapy.
The Phase I clinical trial, a single-center, dose-escalation study, tested QH104 on B7H3-positive rGBM patients who had already completed standard treatments. These patients had a Karnofsky Performance Status (KPS) score of 60 or higher and a minimum life expectancy of three months. The trial followed a “3+3” dose escalation model, with patients receiving intrathecal injections of QH104 once per month.
As of March 30, 2024, seven high-grade rGBM patients (five males, two females, median age 60) had undergone at least one dose of QH104 and were monitored for an average of 6.5 months. The therapy showed an excellent safety profile, with no dose-limiting toxicities. Although minor side effects like fever and headaches were observed, no severe cases of cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), or GvHD occurred. Importantly, QH104 exhibited remarkable efficacy, with an objective response rate (ORR) of 42.9% and a disease control rate (DCR) of 100%, ensuring that all patients saw some level of disease stabilization.
Even more encouraging, 30 days post-treatment, the infused cells were still detectable in patients, indicating the therapy’s persistence. Additionally, a positive correlation between B7H3 expression and clinical outcomes suggests that QH104 could be further personalized to optimize patient selection and treatment protocols in the future.
This pioneering approach marks a significant step forward in CAR-γδT therapy, offering hope for patients with relapsed glioblastoma.
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #Immunotherapy #MedicalBreakthrough #CellTherapy #HealthcareInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Chinese ADC Drug Targeting B7-H3 Approved for Clinical Trials!
Chinese ADC Drug Targeting B7-H3 Approved for Clinical Trials!

ADC
#ADC #Immunotherapy#B7H3 #LungCancer #HeadAndNeckCancer #ADCDrug
On September 20, the China National Medical Products Administration (NMPA) approved BeiGene’s innovative anti-cancer drug, BGB-C354, for clinical trials. This exciting news marks the first approval for clinical research of this novel molecule in China. BGB-C354 is an Antibody-Drug Conjugate (ADC) specifically designed to target B7-H3, a protein often overexpressed in various cancers such as lung cancer, prostate cancer, breast cancer, and squamous cell carcinoma of the head and neck.
BGB-C354 holds significant promise for treating patients with advanced solid tumors, particularly lung cancer and head and neck squamous cell carcinoma (HNSCC). B7-H3 has gained attention as a key anti-cancer target, with its high expression often linked to poor prognosis. By leveraging the power of ADC technology, BGB-C354 aims to selectively deliver potent anti-cancer drugs directly to tumor cells, minimizing damage to healthy cells.
BeiGene has already initiated a Phase 1 clinical trial in the US and Australia, evaluating the safety, tolerability, and preliminary anti-tumor activity of BGB-C354 as a monotherapy and in combination with anti-PD-1 antibody, Tislelizumab. This recent approval sets the stage for the commencement of clinical trials in China.
Globally, B7-H3-targeted therapies are advancing rapidly. Companies like Daiichi Sankyo, Merck, and GSK are pushing the boundaries with their ADCs, entering Phase 2 and 3 clinical trials. The approval of BGB-C354 marks a significant step in advancing cancer treatment options in China, offering new hope for patients with difficult-to-treat cancers.
To assess whether the condition is suitable for clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ClinicalTrials #BeiGene #OncologyBreakthrough #PharmaInnovation #CancerResearch
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breakthrough in China’s CAR-T Therapy: 13-Year-Old Girl Overcomes Lupus, Opening New Hope for a Cure**
**Breakthrough in China’s CAR-T Therapy: 13-Year-Old Girl Overcomes Lupus, Opening New Hope for a Cure**

Lupus
#LupusTreatment #HopeForLupus #lupus #AutoimmuneDisease #ChinaHealthcare #SystemicLupusErythematosus #Erythematosus #patientstory #SLE
In June 2024, 13-year-old Qingqing experienced a major turning point in her life. A year after being diagnosed with systemic lupus erythematosus (SLE), she received innovative CAR-T therapy at Shanghai Children’s Medical Center and successfully achieved disease remission. SLE, often referred to as the “incurable cancer,” is an autoimmune disease that affects millions of people worldwide, severely damaging the brain, lungs, kidneys, and blood system. Even when not in an acute flare, patients often suffer from chronic symptoms such as fatigue, rashes, pain, and fever.
Qingqing’s treatment marks a significant breakthrough in the global fight against SLE. Previously, SLE patients could only rely on lifelong medications, such as steroids and immunosuppressants, to control the disease and prevent severe complications. However, these drugs often come with side effects and long-term organ damage. Qingqing’s case has offered the world new hope. A team led by Dr. Li Benshang, chief physician of the Hematology and Oncology Department, and Dr. Yin Lei, head of the Nephrology Department, pioneered the use of CAR-T therapy in SLE treatment and initiated a clinical study. After just two months of treatment, Qingqing’s condition has fully gone into remission, and all medications were discontinued.
CAR-T therapy was originally used in cancer treatment by extracting a patient’s T cells and genetically modifying them to recognize and kill abnormal cells in the body. In the treatment of SLE, CAR-T therapy eliminates the abnormal plasma cells producing autoantibodies, effectively “resetting” the patient’s immune system and addressing the root cause of the disease.
Qingqing’s success story is not only a milestone in China’s CAR-T technology but also a major leap forward in the global treatment of autoimmune diseases like SLE. Since the first CAR-T treatment cured an SLE patient in 2021, numerous clinical trials have been conducted worldwide, and several Chinese hospitals have also achieved success. However, while early results are encouraging, further research and validation are needed to confirm the long-term safety and efficacy of the treatment.
With 20% of the global population affected by various types of autoimmune diseases, CAR-T therapy holds the potential to bring new life to millions of patients. China’s ongoing research and international collaboration in this field offer unprecedented hope for overcoming persistent diseases like SLE.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
(Https://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#CAR-Ttherapy #LupusTreatment #AutoimmuneDisease #MedicalInnovation #ChinaHealthcare #Immunotherapy #CancerTreatment #HealthBreakthrough #HopeForLupus #FutureOfMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breaking News! China’s Cancer Drug Outperforms Global PD-1 Inhibitor!**
**Breaking News! China’s Cancer Drug Outperforms Global PD-1 Inhibitor!**

PD1
#AK112 #CancerDrug #PD-1 #PD1Inhibitor #CancerTreatment #BispecificAntibody #NSCLC
China’s pharmaceutical industry has reached a historic milestone! Remember AK112 (Ivonescimab), which took center stage during this year’s ASCO conference, attracting global attention for its groundbreaking potential? The long wait is finally over, and the results are in!
In May, Akeso Biopharma, a leading Chinese pharmaceutical company, announced that its independently developed bispecific antibody drug AK112 achieved a major milestone in clinical trials. AK112 demonstrated superior efficacy to the globally renowned PD-1 inhibitor Keytruda (K Drug) in treating PD-L1-positive non-small cell lung cancer (NSCLC), marking a revolutionary breakthrough for China’s innovative cancer treatments.
**Clinical Data that Surprised the World**
During the World Conference on Lung Cancer 2024, Professor Zhou Caicun, one of China’s top lung cancer experts, revealed the results of the HARMONi-2 study, which had the entire audience applauding. The data exceeded expectations, demonstrating that AK112 is not just on par with but superior to international “superstar” cancer drugs in several key areas.
– **Progression-Free Survival (PFS):** AK112 improved PFS by an astonishing 91.4% compared to Keytruda, reaching 11.14 months versus 5.82 months.
– **Risk Reduction:** AK112 reduced the risk of disease progression or death by 49%.
– **Objective Response Rate (ORR):** AK112 outperformed Keytruda with a response rate of 50.0% compared to 38.5%.
– **Disease Control Rate (DCR):** AK112 maintained a significant advantage, achieving 89.9% versus 70.5%.
While AK112 showed slightly higher Grade 3 side effects due to its VEGF inhibition, its overall performance remains remarkable. Although overall survival (OS) data is still maturing, AK112’s impressive PFS results suggest that its survival benefits will likely be just as extraordinary.
**The Power of Bispecific Antibodies**
AK112 is part of a new class of cancer drugs called bispecific antibodies, capable of targeting two different antigens at once. In this case, AK112 targets both PD-1 and VEGF, two well-known lung cancer markers. This dual-target approach offers patients better therapeutic options and increased convenience, potentially replacing the traditional combination of PD-1 inhibitors with anti-angiogenic drugs like Avastin.
This breakthrough has made AK112 the first and only bispecific antibody drug to outperform Keytruda in head-to-head clinical trials, solidifying its place as a future game-changer in cancer treatment.
**A Milestone for China’s Pharma Industry**
In May 2024, AK112 received regulatory approval from China’s National Medical Products Administration (NMPA) for treating EGFR-mutated, locally advanced, or metastatic NSCLC, becoming the world’s first approved PD-1/VEGF bispecific antibody.
This marks a monumental achievement for China’s pharmaceutical innovation. AK112’s success demonstrates that Chinese researchers and drug developers, in just two decades, have achieved what took developed nations over a century. China’s presence at top-tier global cancer conferences like ASCO and ESMO has never been stronger, with clinical data that leaves the world in awe.
China’s pharmaceutical industry may have started late, but its rapid progress is a testament to the ingenuity and hard work of its researchers. Once relying on generics, China is now a leader in pharmaceutical innovation, offering cutting-edge treatments to patients around the globe. The future of cancer treatment is here, and it’s painted in the bold color of “China Red.”
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #MedicalInnovation #PharmaBreakthrough #ChinaPharma #AK112 #LungCancer #BispecificAntibody #PD1Inhibitor #GlobalHealth #MedicalAdvancements #ProudlyMadeInChina #WorldClass
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Breakthrough Chinese Monoclonal ADC Targets Nectin-4: Achieving 91.9% Disease Control Rate Across Multiple Solid Tumors**
**Breakthrough Chinese Monoclonal ADC Targets Nectin-4: Achieving 91.9% Disease Control Rate Across Multiple Solid Tumors**

Monoclonal ADC
#MonoclonalADC #Nectin4 #Solidtumor #9MW2821 #cervicalcancer #urothelialcarcinoma #esophagealcancer #breastcancer
Meet 9MW2821, a groundbreaking monoclonal antibody-drug conjugate (ADC) developed in China that targets Nectin-4, an adhesion molecule highly expressed in several solid tumors, including cervical cancer (CC), urothelial carcinoma (UC), esophageal cancer (EC), and breast cancer. Recently, this innovative therapy was granted Breakthrough Therapy Designation by the China Center for Drug Evaluation (CDE) for treating locally advanced or metastatic urothelial carcinoma, specifically in patients who have previously failed platinum-based chemotherapy and PD-(L)1 inhibitors.
The latest clinical results were unveiled at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, showcasing the remarkable efficacy of 9MW2821 in a Phase 1/2 trial involving 260 patients with various advanced solid tumors. These included UC, triple-negative breast cancer (TNBC), EC, and CC. Among 37 evaluable UC patients, the disease control rate (DCR) soared to an impressive 91.9%, with an objective response rate (ORR) of 62.2%. Additionally, the median overall survival (mOS) reached 14.2 months, while the median progression-free survival (mPFS) was 8.8 months.
This promising therapy is poised to redefine treatment paradigms for patients with advanced solid tumors who have limited options, marking a significant milestone in the global oncology landscape.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#OncologyBreakthrough #CancerTreatment #Nectin4 #ADC #ClinicalResearch #Immunotherapy #UC #TNBC #CancerAwareness #BiotechInnovation
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79
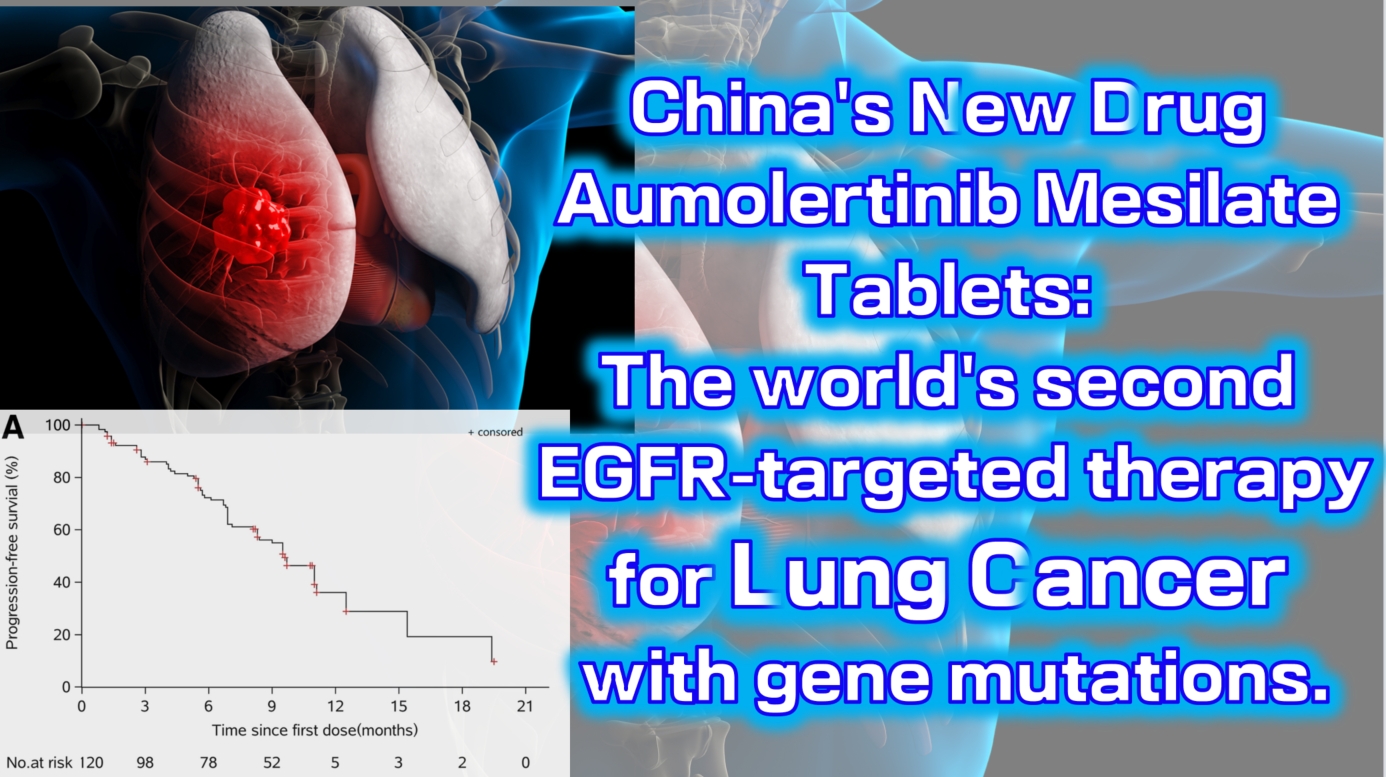
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.
China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.

lung cancer
#LungCancer #EGFRInhibitor #TargetedTherapy #Aumolertinib #NSCLC
Aumolertinib Mesilate Tablets (Ameile) were approved for marketing in China on March 18, 2020. It is China’s first third-generation EGFR-targeted therapy for lung cancer with gene mutations and the second such drug globally, following Osimertinib.
On August 20, 2024, the new indication marketing application for Aumolertinib Mesilate Tablets was accepted for priority review by the China Center for Drug Evaluation (CDE). This indication is for the treatment of unresectable locally advanced non-small cell lung cancer (NSCLC) that has not progressed following platinum-based chemoradiotherapy. This is the fourth indication for which the drug has submitted a marketing application, and it is expected to be approved in the second quarter of 2025.
The Phase 1 clinical trial (NCT0298110) enrolled a total of 120 patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). The results showed an objective response rate (ORR) of 52% (range: 42–63), with a disease control rate (DCR) as high as 92% (range: 84–96). The median progression-free survival was 11.0 months (95% CI: 9.5-not reached).

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#CancerTreatment #ClinicalTrials #PharmaInnovation #Oncology #DrugApproval #Biotech #PrecisionMedicine
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

China’s first BCMA CAR-T therapy successfully treats an overseas patient with advanced relapsed multiple myeloma
China’s first BCMA CAR-T therapy successfully treats an overseas patient with advanced relapsed myeloma.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Https://wa.me/+8613717959070
Email: doctor.huang@globecancer.com
#MultipleMyeloma #CAR-T #CancerTreatment #MedicalBreakthrough #Oncology #HealthcareInnovation #BCMACART
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Multiple Myeloma Solution ** Breakthroughs and Hope in Blood Cancer Treatment**
Multiple Myeloma Solution
** Breakthroughs and Hope in Blood Cancer Treatment**

Mutiple Myeloma
Multiple myeloma is a malignant blood cancer caused by the abnormal proliferation of plasma cells in the bone marrow, often affecting the bones, kidneys, and various organs and systems. With the advancement of modern medical technology, the understanding and treatment of multiple myeloma have greatly improved, bringing unprecedented hope to patients.
### Causes and Risk Factors of Multiple Myeloma
The causes of multiple myeloma are complex, typically involving multiple factors such as genetic mutations, immune system abnormalities, exposure to harmful chemicals, viral infections, and genetic predisposition. Additionally, lifestyle and environmental factors like smoking, obesity, and certain occupational exposures are also believed to increase the risk of developing the disease. With an aging population, the incidence of multiple myeloma is on the rise.
### Evolution of Treatment: Traditional and Innovative Approaches
Although multiple myeloma was once considered a difficult-to-treat disease, recent advancements in treatment methods have made significant progress. Traditional treatments include chemotherapy, radiation therapy, and bone marrow transplantation, which have extended patients’ survival to some extent but come with limited efficacy and significant side effects.
However, with advances in medical technology, new treatment options have emerged. Monoclonal antibodies have played a key role in treating multiple myeloma, targeting cancer cells precisely while reducing harm to healthy cells. Antibody-drug conjugates take this a step further by delivering chemotherapy drugs directly to cancer cells, enhancing efficacy while minimizing side effects.
Small molecule targeted drugs represent another breakthrough. These drugs inhibit specific genes or proteins in cancer cells, preventing their growth and spread. For example, BCL-2 inhibitors and protein kinase inhibitors have shown promising results in clinical trials, offering more treatment options.
### Immunotherapy: Leading the Future of Hope
Immunotherapy is becoming increasingly important in the treatment of blood cancers, especially in the field of multiple myeloma. By activating the patient’s own immune system to attack cancer cells, immunotherapy holds great promise. Among the most forward-looking therapies is CAR-T cell therapy. This treatment involves genetically modifying a patient’s T cells to recognize and kill cancer cells, and it has achieved remarkable results in multiple myeloma patients.
In recent years, China has made significant progress in the research and application of immunotherapy. Particularly in the field of blood cancers, China has developed a comprehensive treatment system with established protocols and consensus. The BCMA-targeted CAR-T cell therapy has shown deep and lasting efficacy in treating multiple myeloma, greatly improving patients’ disease-free survival rates. For instance, China’s fully human BCMA-targeted CAR-T product, Equecabtagene Autoleucel, has demonstrated the best data among CAR-T products for multiple myeloma, with a complete remission rate of 82.4%.
### Looking Ahead
With the continuous emergence of new drugs and therapies, the treatment of multiple myeloma is moving towards individualized and precision medicine. Patients not only experience extended lifespans but also regain their quality of life, returning to normalcy in their everyday lives and work. As medical advancements continue, there is reason to believe that multiple myeloma will no longer be an incurable disease, and every patient will be able to embrace a brighter future.
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070 (Http://wa.me/+8613717959070)
Email: doctor.huang@globecancer.com
#BloodCancerTreatment #CAR_TCellTherapy #CancerBreakthrough #Immunotherapy #BCMACART #MedicalAdvancements #CancerSurvivorship #ChinaMedicalInnovation #HopeForCancerPatients
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Celebrating Discharge: The Joy of a New Life/ Hope /Multiple myeloma
Celebrating Discharge: The Joy of a New Life/ Hope /Multiple myeloma
After a period of treatment, the Singaporean patient Teresa achieved remarkable results with CAR-T therapy at Jiahui International Hospital in Shanghai. Her condition reached complete remission (CR), and to celebrate this great news, the hospital held a brief yet heartwarming celebration for her.
In the celebratory photos, Teresa, along with Dr. Vicky Lee and her team of doctors and nurses, are all smiles, radiating the joy of victory. The photos not only captured this happiness but also served as a testament to the hard work and professionalism of the medical staff. Every member of Dr. Vicky Lee’s team is a true hero, using their expertise and selfless dedication to help Teresa overcome her illness.
Teresa, filled with emotion, said: “Although the treatment process was tough, I felt immense warmth and support from the doctors and nurses at Jiahui Hospital. Their professionalism and care gave me confidence, which ultimately led to such a successful outcome.”
On the day of her discharge, the medical staff extended their heartfelt blessings to Teresa. The nurses kindly reminded her of the precautions she needed to take after leaving the hospital, ensuring she could maintain good health during her recovery at home. Teresa expressed her gratitude to each member of the medical team, thanking them for the care and support they provided when she needed it most.
Standing at the hospital entrance, Teresa looked back on her treatment journey, filled with gratitude and hope. She knew that it was because of the selfless dedication and outstanding professionalism of these medical professionals that she could embrace health once again and look forward to a new chapter in life.
Teresa’s recovery story is not only a personal victory but also the result of the collective efforts of the entire medical staff at Jiahui Hospital. She is deeply grateful for their hard work and believes that many more patients will find new hope for recovery here in the future.
We will continue to follow the patient’s post-treatment progress and provide updates.
#CART #CARTTherapy #Hopeforpatients #FUCASO #Equecel #MultipleMyeloma #jihuiHospital #Shanghai #ChineseCart #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #cancerfight #cancersurvivor #Jiahuihospital
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy
Redefining Hope for High-Risk RRMM Patients Worldwide: A Singaporean Patient`s Journey to China for Groundbreaking CAR-T Therapy

Multiple Myeloma
#MultipleMyeloma #CAR_Therapy #CancerTreatment #HRMM #MM #RRMM #CART
In the fight against multiple myeloma (MM), the last few decades have seen significant advancements, yet the disease remains notoriously difficult to cure, particularly in patients with relapsed/refractory multiple myeloma (RRMM). These patients face enormous challenges, as their options become increasingly limited after multiple lines of therapy have failed. However, hope has emerged in the form of BCMA CAR-T therapy, offering deep remission and long-term survival for those who had nearly lost hope.
One such case is a 58-year-old woman from Singapore, who after exhausting all available treatments in her home country, found new hope in China`s innovative CAR-T therapy. Diagnosed with MM in May 2021 following a month of severe back pain, she underwent a series of treatments including CD38 monoclonal antibodies, immunomodulatory drugs (IMiDs), proteasome inhibitors (PI), and XPO-1 inhibitors. Unfortunately, these therapies failed to halt the progression of her disease, which had become highly resistant to treatment.
In December 2023, she traveled to Beijing Chaoyang Hospital, Capital Medical University, where Professor Chen Wenming took charge of her case. The patient was diagnosed with high-risk MM (IgG-κ type) and admitted to the hospital on November 25, 2023, for CAR-T therapy.
#### The Treatment Journey: A Detailed Overview
Given the patient’s refractory nature and multiple prior treatments, Professor Chen devised a tailored treatment plan to improve her chances of survival and quality of life. In November 2023, her lymphocytes were collected to prepare the CAR-T cells. During this period, she received two cycles of D-PACE (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide) chemotherapy in Singapore to control the extramedullary plasmacytoma.
In February 2024, she returned to Beijing for further evaluation, where her condition was assessed as showing minimal response (MR). She was then administered a lymphodepletion regimen of fludarabine and cyclophosphamide on February 29. The following month, she received a transfusion of BCMA CAR-T cells.
Within five days post-transfusion, the patient developed a fever, which peaked at 39°C. Fortunately, her oxygen saturation and heart rate remained normal, and she was diagnosed with Grade 1 cytokine release syndrome (CRS). After symptomatic treatment, her blood counts recovered by day 15, and she was discharged in stable condition.
Two weeks post-CAR-T therapy, her response was evaluated as a very good partial response (VGPR), and by the two-month mark, her condition had improved to complete remission (CR) with minimal residual disease (MRD) negativity.
#### Insights from Leading Experts
Professor Wee Joo Chng, a specialist in high-risk MM, noted the aggressive nature of the patient’s disease, marked by genetic abnormalities like 1q21+ and t(4;14). Despite the use of multiple potent therapies, including KRd, XVd, and Isa-Pd, the patient’s disease continued to progress rapidly. The emergence of the del(17p) mutation further complicated her prognosis, indicating the need for a novel therapeutic approach.
The FUMANBA-1 study has highlighted the effectiveness of China`s indigenous CAR-T product, Equecabtagene Autoleucel, in achieving deep remission and prolonging survival in RRMM patients. This case demonstrated the therapy`s potential to overcome poor prognostic factors and extend the patient’s survival. Notably, the patient experienced only mild CRS and no immune effector cell-associated neurotoxicity syndrome (ICANS) or infections during treatment. At the two-month follow-up, the patient’s condition had improved to CR with MRD negativity, suggesting that Equecabtagene Autoleucel could be a game-changer for high-risk RRMM patients.
#### A New Frontier in CAR-T Therapy
RRMM patients with double-hit characteristics often experience early relapse and progression, leading to shortened survival times. Traditional therapies, including IMiDs, PIs, and monoclonal antibodies, have failed to overcome these poor prognostic factors, indicating the urgent need for novel treatments. Real-world studies have shown that CAR-T therapy offers comparable progression-free survival (PFS) and overall survival (OS) rates in RRMM patients, regardless of high-risk cytogenetic abnormalities.
The FUMANBA-1 study revealed impressive outcomes for Equecabtagene Autoleucel in RRMM patients, with an overall response rate (ORR) of 98.9% and an MRD negativity rate of 97.8% among CAR-T-naive patients. The CR rate was 82.4%, and 81.7% of patients maintained MRD negativity for over a year.
Globally, four CAR-T products are currently available, and a recent study presented at the 2024 European Society for Blood and Marrow Transplantation (EBMT) compared the short- and long-term efficacy of these therapies. The study’s matching-adjusted indirect comparison (MAIC) analysis revealed that Equecabtagene Autoleucel had a 12-month PFS rate of 94.2%, higher than the 75% observed with Ciltacabtagene autoleucel (CARTITUDE-1 study). Furthermore, the 12-month sustained MRD negativity rate for Equecabtagene Autoleucel was 100%, compared to 53.1% for Ciltacabtagene autoleucel.
These findings suggest that Equecabtagene Autoleucel, a Chinese-developed BCMA CAR-T therapy, offers superior long-term efficacy compared to its U.S. counterpart. As the global community celebrates the first anniversary of its approval, Equecabtagene Autoleucel continues to bring hope to RRMM patients worldwide, further solidifying China’s leading role in the field of cellular therapy.

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp:+8613717959070
Email: doctor.huang@globecancer.com
#ChinaMedicalInnovation #GlobalHealth #MMResearch #PatientCare #InnovativeMedicine #CellTherapy #MedicalBreakthrough
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**Chinese Biopharmaceuticals: Ushering in a New Era in Nasopharyngeal Carcinoma Treatment, EBV-Specific CAR-T Injection Approved for Phase II Clinical Trials in the US and China**
**Chinese Biopharmaceuticals: Ushering in a New Era in Nasopharyngeal Carcinoma Treatment, EBV-Specific CAR-T Injection Approved for Phase II Clinical Trials in the US and China**

Nasopharyngeal Carcinoma
#Nasopharyngeal #Carcinoma #BRG01 #CART #EBVspecific
Biosyngen has announced a milestone achievement: the company’s independently developed BRG01 injection (EBV-specific CAR-T) has received formal written approval from the U.S. Food and Drug Administration (FDA) to conduct pivotal Phase II clinical trials for the treatment of relapsed/metastatic EBV-positive nasopharyngeal carcinoma patients. This is the world’s first original cell drug for solid tumors approved in both the US and China to enter Phase II trials, bringing hope and breakthroughs to the field of solid tumor treatment.
Previously reported, the Center for Drug Evaluation (CDE) of the National Medical Products Administration has agreed to conduct pivotal Phase II clinical trials of BRG01 injection (EBV-specific CAR-T). Patients for the BRG01 US-China Phase I clinical trials completed enrollment by the end of January this year, and all trial participants have received BRG01 reinfusion treatment. The Phase I registered clinical trial of BRG01 has successfully completed DLT (dose-limiting toxicity) observation and efficacy evaluation in 9 patients, all of whom were advanced nasopharyngeal carcinoma patients who had failed at least one treatment, including PD-1 antibody immune checkpoint inhibitors.
The data shows that BRG01 has demonstrated excellent safety and preliminary efficacy: in PET-CT scans, 75% of high-dose patients experienced a local reduction in tumor lesions and a decrease in metabolic activity, with some patients achieving 100% complete remission of tumor lesions. Additionally, BRG01 also demonstrated excellent anti-EB virus efficacy, with a significant reduction of EB virus load in the peripheral blood of patients after reinfusion to normal levels.
These data not only highlight the potential of BRG01 in tumor treatment but also demonstrate its dual advantages in antiviral therapy, laying a solid foundation for future clinical applications. This is believed to be the key reason for the FDA’s approval of BRG01 to enter Phase II clinical trials.
The FDA’s approval not only highly recognizes the preliminary research results of BRG01 injection but also fully affirms Biosyngen’s innovative capabilities and research and development strength in the field of cellular immunotherapy. Biosyngen will take this as an opportunity to accelerate the clinical research process of BRG01 injection, striving to achieve the commercialization of this therapy as soon as possible to bring benefits to nasopharyngeal carcinoma patients worldwide.
Biosyngen’s layout in the field of solid tumor cell therapy goes far beyond this. The company has become a biotech enterprise with three major cellular therapies for both solid and hematological tumors: CAR-T, TCR-T, and TIL. The product pipelines have completed dual submissions and approvals in both the US and China, and the indications for the latter two include a variety of solid tumors such as lung and liver cancer.
Looking to the future, with Biosyngen’s efficient execution and rapid R&D achievements, we have reason to expect more clinical breakthroughs in solid tumor cell drugs in a shorter time, bringing new treatment hopes to patients.
🎉🎉To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
WhatsApp: +8613717959070
Email: doctor.huang@globecancer.com
#Biosyngen #CAR_T #CancerTreatment #NasopharyngealCarcinoma #FDAApproval #ClinicalTrials #MedicalBreakthrough #BiotechInnovation #CancerResearch #Immunotherapy #HealthcareInnovation #CancerHope #SolidTumors #GlobalHealth #CancerCare
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 78
Warning: Trying to access array offset on value of type bool in /www/wwwroot/www.medtourcn.com/wp-content/themes/medical-directory/framework/theme/medicaldirectory-image.php on line 79

**A Groundbreaking ADC Drug for Cervical Cancer Approved in China Macau**
**A Groundbreaking ADC Drug for Cervical Cancer Approved in China Macau**

Cervical Cancer
